Type 1 Diabetes:
Cellular, Molecular & Clinical Immunology
Chapter 9 - Epidemiology
of Type I Diabetes (Revised 11/3/2010)
Marian Rewers, MD, PhD1,
Jill Norris, PhD2, Adam Kretowski, MD, PhD
1Barbara
2Department of Preventive Medicine & Biometrics, UCD, Aurora, CO
Type 1 Diabetes
Type 1 diabetes accounts for about 10% of all diabetes, affecting approximately
1.4 million people in the U.S., and 10-20 million globally (1,2). About 40% of
persons with type 1 diabetes develop the disease before 20 years of age, thus
making it one of the most common severe chronic diseases of childhood. In the
This chapter considers the whole spectrum of type 1 diabetes - from genetic
susceptibility, through ß-cell autoimmunity without diabetes, to clinical type
1 diabetes. Special emphasis is given to epidemiological data relevant to the
primary and secondary prevention.
Natural History of Type 1a Diabetes
The American Diabetes Association and the World Health Organization have
revised the classification of diabetes mellitus (9,10).
The new classification of diabetes is now primarily based on pathogenesis
rather than on the requirement for insulin therapy. Based on the new system, type 1 diabetes (previously defined as
insulin-dependent or juvenile diabetes) is caused by ß cell destruction, often
immune mediated, that leads to loss of insulin secretion and absolute insulin
deficiency. The etiologic agents that cause the autoimmune process
and ß cell destruction are not well established. Type 1 diabetes also includes
cases that are thought not to be immune mediated, but are characterized by
absolute insulin deficiency (insulinopenia).
The current classification of diabetes mellitus distinguishes type 1a
(autoimmune) (11)
and type 1b (not immune-mediated) form that remains poorly defined (12,13).
Type 1a is the most common form of diabetes among children and adolescents of
European origin, usually characterized by acute onset and dependence on
exogenous insulin for survival. In adults, the disease is nearly as frequent as
in children, but often less dramatic onset may lead to misclassification as
type 2 and a delayed insulin treatment. About 60% of persons with type 1
diabetes are diagnosed as adults.
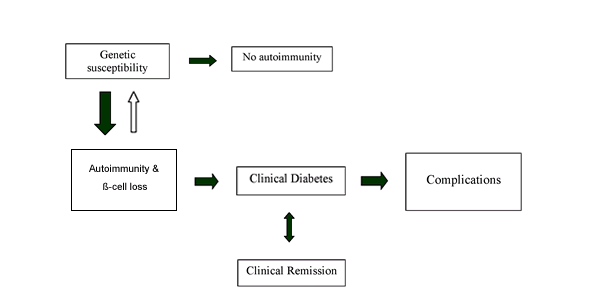
The natural history of type 1a diabetes (Figure 1) includes four distinct
stages:
1) pre-clinical ß-cell autoimmunity with progressive defect of insulin
secretion;
2) onset of clinical diabetes;
3) transient remission; and
4) established diabetes associated with acute and chronic complications and
premature death.
B-cell autoimmunity
In most patients, the etiology of the autoimmune
process and ß-cell destruction is not known (14).
The process is mediated by macrophages and T lymphocytes with detectable
autoantibodies to various ß-cell antigens. Currently, autoimmunity is defined
by the presence of autoantibodies (11), because their measurement is reliable
and standardized across laboratories, in contrast to the cellular markers. The
initial islet cell antibody (ICA) assay, using immunofluorescence and
pancreatic tissue (15) has been notoriously difficult to standardize and has
been replaced by a combination of specific ß-cell autoantibodies to insulin
(IAA) (16,17,18),
glutamic acid decarboxylase (GAD) (19,20), and tyrosine phosphatase ICA512
(IA-2) (21). These tests have been shown to be quite sensitive and predictive
in relatives of type 1 diabetes patients (22,23)
and in the general population (24).
However, to avoid false or only transiently positive results duplicate testing
and independent sample retesting should be considered in the prediction
strategy (25,26,27,28).
Progression from ß-cell autoimmunity to
clinical diabetes
The duration of pre-clinical ß-cell autoimmunity is variable and precedes the
diagnosis of diabetes by up to 13 years (29,30). In persons with persistent
autoantibodies, there is an early loss of spontaneous pulsatile insulin
secretion, progressive reduction in the acute insulin response to intravenous
glucose load, followed by decreased response to other ß-cell secretagogues,
impaired oral glucose tolerance and fasting hyperglycemia (31). However, a
non-progressive ß-cell defect has been shown to exist for many years in
monozygotic twins and other relatives of type 1 diabetes persons. The recent
studies show that detailed characterization of islet autoantibodies that
include determination of titer, epitope specificity, IgG subclass and affinity
would improve diabetes prediction in autoantibody-positive relatives and
subjects at risk from general population (32,33,34).
The highest risks for progression to type 1 diabetes is associated with
high-titer IA-2A and IAA, IgG2, IgG3, and/or IgG4 subclass of IA-2A and IAA,
and antibodies to the IA-2-related molecule IA-2beta (32,35,36).
Using models based on these antibody characteristics, autoantibody-positive
relatives can be classified into groups with risks of diabetes ranging from 7
to 89% within 5 years (36).
Moreover IAA affinity, measured by competitive radiobinding assay, can further
stratify the risk of type 1 diabetes development (36,37,38).
IAA affinity in multiple antibody-positive children is on average 100-fold
higher than in children who remained single IAA positive or became autoantibody
negative. All high-affinity IAAs require conservation of human insulin A chain
residues 8-13 and are reactive with proinsulin, while lower-affinity IAAs are
dependent on COOH-terminal B chain residues and do not bind proinsulin. 92% of
children who developed multiple islet autoantibodies or diabetes are correctly
identified by high-affinity IAA and 82% who did not develop multiple islet
autoantibodies or diabetes are correctly identified by low-affinity IAA (36).
Studies in the first-degree relatives of type 1 diabetes patients (30,39,40)
and in school children with no family history of type 1 diabetes (41,42,43,44,45)
have reported
ß-cell autoimmunity may remit and reappear in the course of viral infections or
variable exposure to dietary causal factors (26,27). The cumulative ß-cell
damage and increases in insulin resistance with obesity and physical
inactivity, may eventually cause diabetes at a later age. Those people in whom
the disease process is slow may present with type 1 diabetes as adults, develop
diabetes that does not require insulin treatment, or may even escape diabetes
altogether. Markers of autoimmunity can be detected in 14-33% of diabetes
patients classified on clinical grounds as “Type 2” (48,49) and are associated
with early failure of oral hypoglycemic drug therapy and insulin dependence in
these patients. A term “latent autoimmune diabetes of adults” (LADA) (50) has
been coined for this slowly progressing form of type 1 diabetes.
Clinical Onset of Type 1 Diabetes
Most epidemiological data on type 1 diabetes are based on a clinical, standard
definition that has been widely used for a long time: 1) physician diagnosis of
diabetes , 2) daily insulin injections instituted at the time of diagnosis, and
3) residence by the patient in the area of registration at the time of first
insulin administration. This practical definition is not taking into account
the nuances of etiological classification into Type 1a, 1b, and other forms of
diabetes.
In industrialized countries, 20-40% of type 1 diabetes patients younger than 20
years presents in diabetic ketoacidosis (DKA) (51,52,53,54). Younger age,
female gender, HLA-DR4 allele (55), lower socioeconomical status and lack of
family history of diabetes have been associated with more severe presentation.
Younger children present with more severe symptoms at diagnosis, because
children younger than 7 have lost on average 80% of the islets, compared to 60%
in those 7-14 year old and 40% in those older than 14 (56). Case fatality in
industrialized countries ranges between 0.4-0.9% (57). While brain edema is
believed to be the major cause of onset death, the risk factors are poorly
understood and heterogeneous.
Both DKA and onset deaths are largely preventable, because most of the patients
have typical symptoms of polyuria, polydipsia, and weight loss for 2-4 weeks
prior to diagnosis. The diagnosis is straightforward in almost all cases, based
on the symptoms, random blood glucose over 200 mg/dl and/or HbA1c >7%. Oral
glucose tolerance test (OGGT) is rarely needed for diagnosis. In questionable
cases, negative OGGT allows ruling out current diabetes and, in combination
with negative ß-cell autoantibodies, offers reassurance that diabetes is not
around the corner. Traditionally, nearly all children with newly diagnosed type
1 diabetes were hospitalized. More recently, an increasing proportion of these
children have been managed on an outpatient basis, especially in urban centers
with specialized diabetes education and treatment facilities. In Colorado, USA,
the proportion of children receiving only outpatient care at onset increased
from 6% in 1978 to 35% in 1988 (58) and 75% in 2000. Hospitalization at onset
does not improve short-term outcomes such as readmission for DKA or severe
hypoglycemia (51,54), if adequate family education and follow-up is available
on outpatient basis. Onset hospitalizations and subsequently acute
complications have similar predictors: biological (younger age, lower
endogenous insulin secretion) and psychosocial (lower socioeconomic status, limited
access to health care, dysfunctional family).
Prevalence of type 1 diabetes
Initial examination of a disease generally begins with cross-sectional data on
its prevalence (i.e., the number of people in the population who have the
disease at a given point in time). Selected estimates of type 1 diabetes
prevalence in different populations with at least 90% ascertainment of cases
are shown in Table 9.1 (see the end of the chapter).
The prevalence of type 1 diabetes in children aged less than 15 years ranges
from 0.05 to 0.3% in most European and North American populations (59).
Comparisons of prevalence data may be subject to considerable bias, since
prevalence is determined not only by disease incidence, but also by case
survival, which may vary markedly across populations. Prevalence data, however,
are useful in determining the public health impact of type 1 diabetes. For
example, in the early 1990's, the number of type 1 diabetic patients 0-19 years
of age in
Incidence of type 1 diabetes
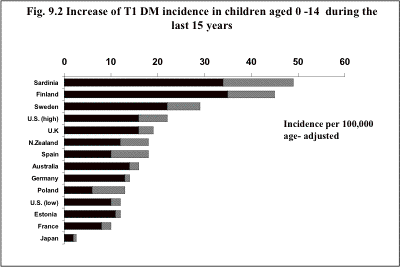
Geographic location: One
of the most striking characteristics of type 1 diabetes is the large geographic
variability in the incidence (61,62,63)
(Fig.9. 2). Scandinavia and the Mediterranean
In the general population, the prevalence of ß-cell autoimmunity appears to be
roughly proportional to the incidence of type 1 diabetes in the populations
(64,65). In contrast, the prevalence of ß-cell autoimmunity in first-degree
relatives of type 1 diabetic persons does not differ dramatically between high
and low risk countries. The incidence of ß-cell autoimmunity is higher in
relatives younger than 5 years (3.7%/yr), compared to those 5-9 yr old
(0.5%/yr) (66). During 5 years of follow-up, none of the relatives older than
10 has developed ß-cell autoimmunity (66), suggesting that ß-cell autoimmunity
develops primarily before the age of 5 and that it may remit in many cases
(44,45).
The clinical picture of the disease is similar in low- and high-risk areas
(67,68), making it unlikely that the inter-population difference is due to
misclassification of different types of diabetes. However, in many populations
(69,70)
type 2 diabetes is an increasing or already the predominant form of diabetes in
children (71)
making correct diagnosis and treatment increasingly difficult.
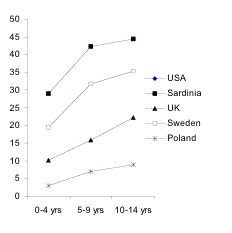
Age: Type 1 diabetes incidence peaks at the ages of 2, 4-6
and 10-14 years, perhaps due to alterations in the pattern of infections or
increases in insulin resistance. Recently published data suggest that in many
populations the highest rate of incidence is observed in the 10-14 age group,
while the highest annual increase is in the 0-4 yrs age children (Figure
9.2.1). The age-distribution of type 1 diabetes onset is similar across
geographic areas and ethnic groups (68) (see also Figure 3). There have been
only a few studies that have examined the incidence of type 1 diabetes in
adults, mainly because of the difficulty of distinguishing type 1 diabetes from
insulin-requiring type 2 diabetes in older individuals. The incidence decreases
in the third decade of life (72,73,74), only to increase again in the fifth to
seventh decades of life (75,76). It has been speculated that the incidence of
type 1 diabetes increases again in the fifth through seventh decades of life
(39,40), but there is no hard evidence of such increase, and it is not known
whether there are etiologic differences between childhood- and adult-onset type
1 diabetes.
|
|
|
Figure 9.2.1. Current (1988-2002)
trends in incidence of type 1 diabetes (A) and rates of annual incidence
increase (B) in different age groups: 0-4, 5-9 and 10-14 yrs.
The prevalence of
Race and ethnicity: The
incidence data from early 1990 suggested a significant racial difference in
type 1 diabetes risk in multiracial populations, although not of the same
magnitude as the geographic differences (Table 9.2). In the U.S., non-Hispanic
whites were about one and a half times as likely to develop type 1 diabetes as
African Americans (79) or Hispanics (80) (Figure 9.3). This was similar to the
differences reported from Montreal, where children of British descent had about
one and a half the risk of type 1 diabetes in children of French descent (84)
(Table 9.2).
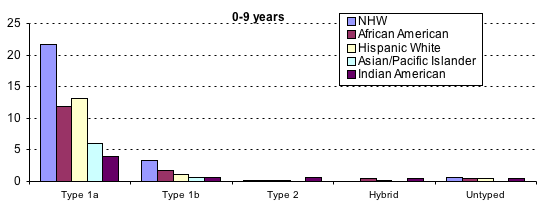
T1a=positive auto-antibodies
T1=insulinopenia without auto-antibodies
T2=high insulin secretion without auto-antibodies
Hybrid=features of both T1a and T2
Untyped=preserved insulin secretion without auto-antibodies
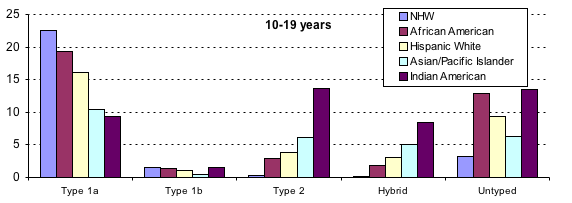
NHW=Non-Hispanic
White
AA-African American
H-Hispanic
API-Asian/Pacific Islander
AI-America Indian
Figure 9.2.2. Epidemiology of
diabetes in children and adolescents 0-9 yrs and 10-19 yrs old in
Interestingly, recent data from the SEARCH
study have shown a rising trend in type 1 diabetes not only in non-Hispanic
white population, but also among Hispanic and African American children (Figure
9.2.2) (81). In fact, however the number of new type 1 diabetic patients of
African-American origin aged 10–14 years has risen significantly during the
last 10-15 years and the incidence of type 1 diabetes in this age group is
almost equal in the white and African-American populations, it is still almost
twofold difference between non-Hispanic white and African-American children in
the incidence of type 1 type in younger children (0-9 yr) (81,82). One could
suspect that the marked increase in incidence in the African-American
population may be in part due to misclassification of cases actually having
type 2 diabetes, as there is an epidemic of type 2 diabetes in children in the
U.S., which largely affects African-American children over the age of 10 years
and many cases of type 2 diabetes require treatment with insulin at the time of
diagnosis. It is unlikely, however, that the increased incidence of type 1 diabetes
in African-American children can be explained only by misclassification. The
diagnosis type 1 diabetes in the SEARCH study was confirmed by the presence of
insulinopenia and diabetic auto-antibodies (81). Similarly to this observation,
the high annul increase in the incidence of type 1 diabetes has been recently
reported among the children of south Asian immigrants (Indian, Pakistani,
Bangladeshi) in
|
Location |
Incidence |
95% Confidence Interval |
|
|
|
|
|
White |
16.2 |
14.1-18.4 |
|
Black |
11.8 |
7.9-17.2 |
|
|
|
|
|
Non-Hispanic White |
16.4 |
15.0-17.8 |
|
Hispanic |
9.7 |
7.4-12.4 |
|
|
|
|
|
French |
8.2 |
7.5
- 8.9 |
|
British |
15.3 |
12.8-17.8 |
|
Italian |
10.7 |
7.5-13.9 |
|
Jewish |
17.2 |
10.4-24.0 |
|
Other |
13.1 |
10.9-15.3 |
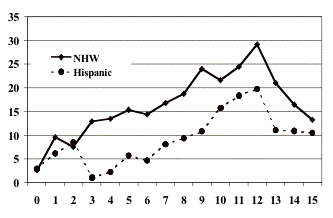
Figure 9.3. Incidence of T1 DM in
Little data concerning ß-cell autoimmunity
is available for non-Caucasian populations. Among 86,000 relatives of type 1
diabetic patients screened for the DPT-1 trail, lower prevalence of
Gender: In general,
males and females have similar risk of type 1 diabetes (86),
with the pubertal peak of incidence in females preceding that in males by 1-2
years. In lower-risk populations, such as
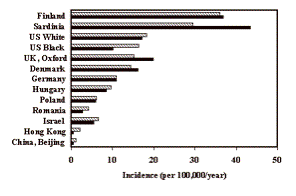
Figure 9.4. Gender
differences in the incidence of type 1 diabetes.
Type 1 diabetes diagnosed in adulthood seems
to be associated with male excess with a male:female ratio between 1.3 and 2.15
in most populations of European origin (91,92,93,94,95),
but there is considerably less information concerning type 1 diabetes with
onset in adult life. These findings contrast with data from animal models of
type 1 diabetes (the NOD mouse), in which diabetes progression is almost twice
as common in females (96). Several pathways for these differences have been
explored, such as the effects of sex hormones, hormone substitution, and
pregnancy on autoimmunity, the relationship between genetic risk factors for
diabetes and sex (97),
but the reasons for these differences are yet not known.
Time: The incidence of
type 1 diabetes varies markedly over time, both seasonally and annually. In the
Northern Hemisphere, the incidence declines during the warm summer months;
similarly in the Southern Hemisphere, the seasonal pattern exhibits a decline
during the warm months of December and January, implicating a climatic factor
(98). This seasonal pattern appears to occur only in older children (99,100),
suggesting that factors triggering diabetes may be related to school
attendance. The observed seasonality does not appear to be an artifact of
health care seeking or access, but the seasonal patterns differ by the HLA-DR
genotype (101,102).
Most population-based registries have shown an increase in type 1 diabetes
incidence over time (103,104,105,106,107,108).
Periodic outbreaks, sometimes of pandemic proportion, e.g., during 1984-86
(104) appear to be superimposed on a steady secular increase in incidence.
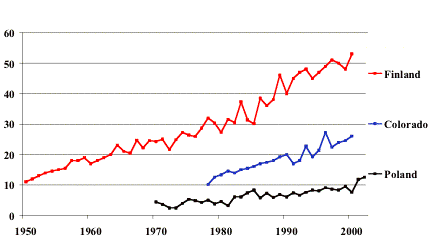
Figure 9.5. Type 1 diabetes incidence
is rising 3-5% /year in different geographic region
with different incidence rate.
While the increase in type 1 diabetes
incidence has affected all age groups, several studies reported particular
increase among the youngest children (109,110,111,112,113).
No reliable information is available concerning potential seasonal or annual
variation in the incidence of ß-cell autoimmunity.
Genetic models are unable to explain the apparent temporal changes in the
incidence (114).
Few studies have analyzed time trends using modeling procedures, looking
specifically at the effects of age, calendar period, or birth cohort on the
incidence of type 1 diabetes (115,116,117,118).
Figure 9.5 displays examples of different types of changes in the incidence of
childhood onset type 1 diabetes that have been observed from late 1960s to mid
1980s: periodic outbreaks superimposed on a steady secular increase (
A recent analysis of data on published incidence trends showed that the
incidence of type 1 diabetes is globally increasing by 3.0% per year, and that
the incidence of type 1 diabetes will be 40% higher in 2010 than in 1998 (119).
The polio model, where autoimmune diabetes results from delayed exposure to
infection that is benign when encountered in early childhood could explain the
recent increase in the incidence but not the shift in diagnosis to earlier
ages. Alternative explanation invokes the congenital rubella model where
increased hygiene has led to a decline in herd immunity to common infections
among women in child-bearing age (120).
These women are more likely to develop viremia during pregnancy resulting in
congenital persistent infection of ß-cells and early onset type 1 diabetes in
the offspring. This model could explain both the increasing incidence of
diabetes and the decreasing age of disease onset.
Genetic factors
Family history of type 1 diabetes:
In moderate type 1 diabetes risk areas, such as the
The risk of ß-cell autoimmunity is higher, because not all autoantibody
positive children develop diabetes by the age of 20. Prevalence estimates from
cross-sectional studies shown in Table 9.1 are obviously less precise than
cumulative incidence rates available for clinical diabetes.
|
Risk Group |
Type 1 Diabetes |
Pre-Diabetic Autoimmunity |
|
General Population |
|
|
|
Family Members |
|
|
Table 9.3. Risk by the age of 20
years of type 1 diabetes and beta-cell autoimmunity in the general population
and family members of type 1 diabetic patients.
'Familial' cases represent about 10% of type
1 diabetes and do not appear to be etiologically different from 'sporadic'
cases in terms of the HLA-DR, DQ gene frequencies, seasonality of onset and
prevalence of islet autoantibodies (126). 'Familial' cases tend to have lower
HbA1c and higher C-peptide levels than 'sporadic' cases, because relatives
recognize diabetes symptoms earlier, however, these differences disappear soon
after diagnosis.
Candidate genes: The
primary loci of genetic susceptibility to type 1 diabetes have been mapped to the
HLA-DR, DQ (127,128,129,130)
and recently also to the DP region (131).
While 50 percent of non-Hispanic whites in the
No particular HLA type seems to be associated with ß-cell autoimmunity,
although associations between different patterns of insulin, IA-2A or GAD
autoantibodies and HLA-DR, DQ phenotypes have been reported (135,136,137).
In the DAISY study HLA DR3/4-DQ8 genotype predicted both persistence of
autoantibodies and progression to diabetes among young first degree relatives
of T1D patients and infants identified by newborn screening for HLA genotypes
associated with diabetes (136). Significant difference in the prevalence of
IAA, IA-2A, GADA and multiple autoantibodies has been observed between siblings
of T1D children with the strongest susceptibility DR3/DR4 genotype and those
with alleles other then DR3 and DR4 (136,137). 90% of the first-degree
relatives (137) and general population children who stay persistently
autoantibody positive (41,138)
express the HLA-DRB1*04, DQB1*0302.
Data from the Type 1 Diabetes Prediction and Prevention Study have shown that
titres of
The HLA-DR2, DQB1*0602 haplotype, which almost completely protects from type 1
diabetes (127), is found in about 15% of GAD and IAA positive young relatives
of type 1 diabetes patients (135,136,137). None of the sibling, taking part in
the DIPP study, who carried DQB1*0602 allele, had IAA, IA-2A or multiple
antibodies. The frequency of children tested positive for
Persistent islet autoimmunity was also found to be associated with non-HLA
genes: insulin gene (INS-23Hph1 polymorphism in children with DR3/4 genotype)
and genes coding cytokines involved in the Th1 regulatory pathway: IL-13, IL-4,
IL4 receptor; but not with CTLA-4 SNPs (140).
Candidate genes outside the HLA region are being identified (141,142,143,144,145,
146,147).
Genes encoding proteins involved in T-cell activation: CTLA-4, PTPN22, VNTR of
insulin gene have been reported to have the functional variants associated with
type 1 diabetes and contribute in 5-10% to genetic risk (148,149,150,151,152).
It is increasingly apparent that the identification of true genetic
associations in common multifactorial disease will require studies comprising
thousands rather than the hundreds of individuals employed to date (153).Perhaps
even more subjects with ß-cell autoimmunity need to be genotyped to precisely
determine the role of HLA and additional type 1 diabetes candidate genes in the
initiation of autoimmunity and progression to diabetes.
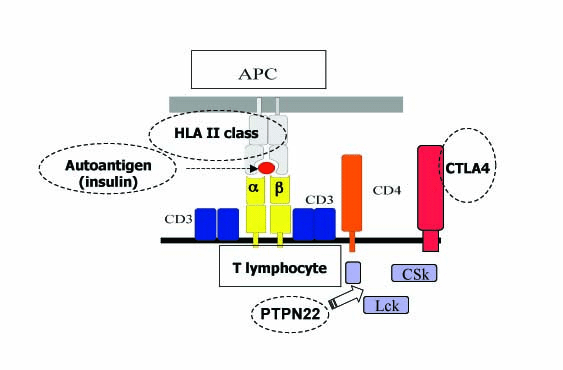
Figure 9.7. Genes encoding proteins
involved in T-cell activation (HLA II class genes, CTLA-4, PTPN22, VNTR insulin
gene) play a key role in type 1 diabetes.
Environmental factors
Twin (154) and family studies indicate that genetic factors alone cannot
explain the etiology of type 1 diabetes. Seasonality, increasing incidence and
epidemics of type 1 diabetes as well as numerous ecological, cross-sectional
and retrospective studies suggest that certain viruses and components of early
childhood diet may cause type 1 diabetes (155).
Viruses: Herpesviruses
(156,157), mumps (158,159), rubella (160,161), retroviruses (162,163), and
rotavirus (164)
have been implicated. Viral infections appear to initiate autoimmunity rather
than precipitate diabetes in subjects with autoimmunity. Two or more infections
with similar viruses may be needed - mice persistently expressing a viral protein
in the ß-cells do not develop ß-cell autoimmunity unless exposed to the same
virus later in life (165,166).
An increased incidence of type 1 diabetes in patients with congenital rubella
syndrome (CRS) is particularly interesting. While CRS is responsible for a
minute proportion of type 1 diabetes and there is little evidence that
postnatal rubella exposure to the wild strain (167) or to the MMR vaccine (170)
causes type 1 diabetes, CRS provides an example of viral persistence leading to
type 1 diabetes. The incubation period of type 1 diabetes in CRS patients is
5-20 years (161) and persistent rubella virus infection of the pancreas has
been demonstrated in some cases. While CRS is not associated with particular
HLA-DR alleles, the distribution of the HLA-DR3 and 4 alleles among patients
with CRS and diabetes resembles that in non-CRS type 1 diabetes patients (160).
Finally, a molecular mimicry has been reported between a rubella virus protein
and a 52 kD ß-cell autoantigen (171).
The evidence is strongest for picornaviruses, which include human
(enteroviruses and rhinoviruses) and animal pathogens (e.g., mouse EMC virus
and Theiler's virus). Enteroviruses have been most strongly linked to human
type 1 diabetes, but convincing proof of causality remains elusive (for review
see 172,173).
Case and autopsy reports (174,175), epidemics of type 1 diabetes associated
with concurrent epidemics of enteroviruses (176,177) and multiple
cross-sectional seroepidemiological studies (172) have been suggestive, but not
entirely convincing. At least 90% of type 1 diabetes patients demonstrate
prolonged period of ß-cell autoimmunity that is hardly compatible with an acute
cytolytic enteroviral infection being a major cause. Enteroviral infection
could, however, initiate ß-cell autoimmunity through molecular mimicry between
CBV P2-C protein and GAD (178)) or a persistent ß-cell infection with
impairment of insulin secretion and expression of self-antigens.
Cross-sectional studies of anti-Coxsackie antibodies in ß-cell autoimmunity
have been week and inconclusive (179) and have been recently replaced by studies
based on detection of picornaviral RNA in bodily fluids using PCR. Prospective
studies of non-diabetic relatives and general population children found a
strong relation between enteroviral infections, defined by PCR, and development
of islet autoantibodies in Finland (180,181,182)
but not in the U.S (616). Studies from
According to polio hypothesis low rate of enterovirus infections in background
population is the reason that young infants may have increased susceptibility
to the diabetogenic effect of enteroviruses, as they are not protected by
maternal or their own antibodies (185,186,187,188).
In line with this hypothesis there are recent observations from
There is accumulating evidence that mechanism of viral infection leading to
b-cell destruction may be related to the induction of interferon a (189,190).
Significant overexpression of INF-a in the pancreatic islets and higher serum
levels INF-a have been documented in newly diagnosed patients with type 1
diabetes (189).
It is also known that INF-a can decrease insulin synthesis/secretion, induce
B-cell apoptosis and has a strong influence on innate immune system (190,191).
Viral infections through the generation of double-stranded RNA may induce INF-a
production and by a direct cytolytic effect on pancreatic B-cells or indirect
activation of innate immune system could trigger of autoimmunity in genetically
susceptible individuals (G*). It was recently demonstrated that poly I:C
(polyinosinic: polycytidylic acid) – a synthetic double-stranded RNA –
stimulates INF-a and induces autoimmune diabetes mainly by activation of
toll-like receptors, and its effect on an immune response is related the
genetic background in different animal models (191).
Additional factors (186,192,193,194,
195)
and season of birth (196)
have been associated with type 1 diabetes.
Possible protective effect of
infections: In animal models, viral infection may protect the
host from developing type 1 diabetes (187,188).
Evidence for such a protective effect in humans still needs confirmation (200,201,202).
According to so called “hygiene hypothesis” infectious agents could have a
protective effect on autoimmunity development (203).
The beneficial mechanisms of an early exposure (in utero or during the first weeks/months of life) of viral,
bacterial or parasitic antigens are not known, however the role of the
regulatory immune CD4 T cells stimulation, toll-like receptors and
superantigens (by activation of T cell clones expressing specific receptor V
gene) is discussed (203).
This hypothesis is supported by the evidence that multiple viral infections
prevent autoimmune diabetes in NOD mouse and by the numerous studies showing
that improvement of socio-economic status, higher level of sanitation and
better medical care (for example: use of antibiotics) is associated with
increased incidence of type 1 diabetes in humans (200,201,202).
Routine childhood immunization:
None of the routine childhood immunization have been shown to increase the risk
of diabetes (170,202,204,205)
or pre-diabetic autoimmunity (206,207,
208).
Dietary factors: Cow's
milk or wheat introduced at weaning trigger insulitis and diabetes in animal
models (209) perhaps through a molecular mimicry (210). Human data are
conflicting, but predominantly negative (211,212,213,214,215,216,217).
An ecological study suggested an association between decrease in breast-feeding
and increase in type 1 diabetes incidence between 1940 and 1980 (218).
Subsequent case-control studies have shown a negative (219,220), positive (221)
or no association (222,223,224). Certain studies (219,225,226) but not others
(221,223,224)
suggested a dose-response relationship between the duration of breast-feeding
and protection from type 1 diabetes.
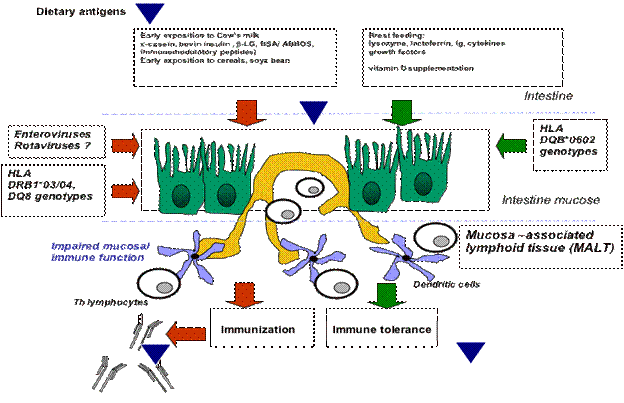
Figure 9.8. Potential mechanisms of
the association between infant diet and beta-cell autoimmunity. (I tried to
change the text in this figure, but could not – is there a way for someone else
to do it? I would change exposition to
exposure (in two places above).
A
meta-analysis found a 50% increase in type 1 diabetes risk associated with a
breast-feeding duration of less than 3 months, and exposure to breast-milk
substitutes prior to 3 months of age (226), but a subsequent meta-analysis
reported much lower risk estimates, and suggested that these findings may be
false positives due to study bias (227).
Breast-feeding may be viewed as a surrogate for the delay in the introduction
of diabetogenic substances present in formula or early childhood diet. More recent
cohort studies failed to find an association between breast-feeding and age at
introduction of cow’s milk and beta-cell autoimmunity (207,215,217,
230, 231, 519). Preliminary data from the Finnish TRIGR study suggests that
incidence of ICA antibodies is significantly lower in children fed until the
age of 6-8 months with the casein hydrolysate in comparison to the group with
conventional cow's milk-based formula (186). Interestingly, another study from
In addition to breast milk substitutes,
such as infant formulas, the infant is exposed to other dietary antigens in the
first year of life that may impact oral tolerance. In the
In the BB
diabetes-prone rat, gluten precipitates the onset of IA (520). Macfarlane et al. identified a wheat storage
protein called glb1 that may be associated with islet damage, by observing that
antibodies to this protein were detectable in patients with diabetes but not in
nondiabetic patients (521). Moreover,
the timing of introduction of cereals (and/or gluten) during infancy, has been
examined in all three prospective studies of the development of IA. Both
BABYDIAB and DAISY have shown an increased risk for IA associated with exposure
to cereals prior to the third month of life when compared with introduction in
the 4th to 6th month of life. Norris et al. found that the timing of introduction of any type of cereal
was associated with an increased IA risk and also found that there appears to
be a U-shaped relationship between risk and age at introduction, the nadir of
the curve occurred with introduction in the 4th to 6th
months of life (231). In contrast, Ziegler et
al. showed the association with gluten specifically and found that a
further protective effect was conferred if foods containing gluten were
introduced after the 6th month (232). It is important to note that
both studies found that the introduction of cereals at less than 3 months of
age resulted in the highest relative risk, particularly in those with the high
risk HLA-DR3/4, DQB1*0302 genotypes (Figure 9.9). These data suggest that there
are specific times in infancy wherein exposure is associated with an increased
risk of developing IA. The risk associated with early exposure may suggest a
mechanism involving an aberrant immune response to cereal antigens in an
immature gut immune system among susceptible individuals.
|
BABY Diab |
DAISY |
Figure 9.9.
Timing of the introduction of solid cereals into infant diet
and the risk of islet autoantibodies.
When examining the role of cereals along with the
timing of introduction, it is also important to consider whether the increased
IA risk is associated with one specific antigen (gluten for example), or if it
is associated with general antigenic stimulation arising from exposure to an
assortment of food antigens. It is interesting that Norris et al. found an effect of timing of cereal introduction in both
gluten-containing and non-gluten-containing cereals (231) whereas Ziegler et al. found the association in
gluten-containing solid foods but not in non-gluten-containing solid foods
(232). Given the difference in the defined dietary variables (the non-gluten
containing food variable in Ziegler et
al. contained non-cereal foods) it is difficult to determine whether the
two studies actually contradict each other. Interestingly, the Finnish
prospective study (DIPP) did not find an association between the timing of
introduction to wheat-based foods and the development of IA. However, to further support the idea that
general antigenic stimulation is more important than the actual antigen in this
disease process is the finding in the DIPP study that early introduction of
fruits, berries and root vegetables was associated with increased risk for IA (519).
Interestingly, Norris et al. found evidence that a child who is still breast feeding at
the time of introduction to cereals has a reduced risk of IA regardless of the
timing of cereal introduction (231). A
similar protective relationship between breast feeding and introduction of
gluten has been observed in celiac disease (525). These findings suggest that
while not protective independently, breast feeding may be a protective mediator
in the relationship between other dietary factors, including but not limited to
cereals, and IA.
Vitamin D3 supplementation
There is increasing evidence that vitamin D3 might contribute to pathogenesis
and prevention of type 1 diabetes. Active vitamin D3 prevents type 1 diabetes
in animal models, modifies T-cell differentiation, modulates dendritic cell
action and modulates cytokine secretion, shifting the balance to regulatory T
cells. It seems possible that birth seasonality in children and/or the presence
of seasonal pattern at diagnosis of type 1 diabetes could be explained by
variation in endogenous vitamin D production during different year season (236,237).
The monthly averages of maximal daily temperature and daily hours of sunshine
were inversely related to the number of new patients per month in
Multiple
studies have examined the role of vitamin D in the pathogenesis of type 1
diabetes. The EURODIAB multi-center case-control study found that diabetic
children were less likely to have been given vitamin D supplements in infancy
than control children (522). This finding is similar to
that found in the previously described case-control study form
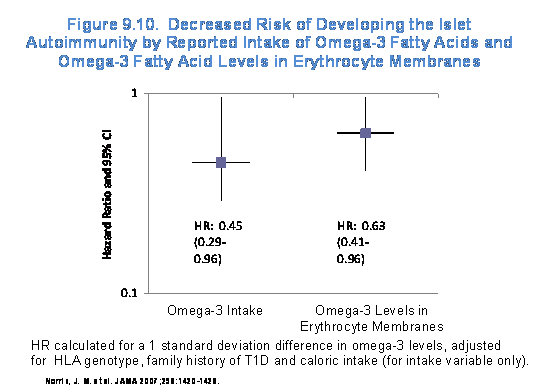
Investigators in DAISY reported that higher
omega-3 fatty acid intake during childhood was associated with a lower risk of
islet autoimmunity (Hazard Ratio [HR]:
0.45), and likewise, that higher omega-3 fatty acid levels in the
erythrocyte membrane were associated with a lower risk of islet autoimmunity
(HR: 0.63) (523) (Figure 9.10).
Chemical compounds: Streptozotocin (239,240) or dietary nitrates and
nitrosamines (241) induce ß-cell autoimmunity in animal models. Circumstantial
evidence suggests a connection between type 1 diabetes and consumption of foods
and water containing nitrates, nitrites or nitrosamines (242,243,244). Multiple
hits of dietary ß-cell toxins may render genetically resistant individuals
susceptible to diabetogenic viruses leading to type 1 diabetes (245).
Weight gain, insulin resistance - “ the
accelerator hypothesis”: It has recently been hypothesized that
excess weight gain and increase in insulin resistance in early childhood is a
trigger event, which initiates the autoimmunity leading to b-cell destruction
and type 1 diabetes development (246,247).
The rising blood glucose (glucotoxicity) accelerates beta-cell apoptosis
directly or by inducing beta-cell immunogens in genetically predisposed
subjects. This so called 'Accelerator Hypothesis' seems to be supported by
several epidemiological case-control and population based cohort studies (246,248,249,250).
A study from
The Environmental Determinants of
Diabetes in the Youth (TEDDY): To resolve the controversy about
the role of environmental factors in the pathogenesis of type 1 diabetes -The
Environmental Determinants of Diabetes in the Youth (TEDDY), a large
international project, with the aim to evaluate the putative environmental
triggers during the 15-year follow-up of several thousand newborn babies
identified with HLA-DR, DQ genotypes associated with type 1 diabetes, has
recently been initiated (155).
Gene-environment interactions:
Type 1 diabetes is likely caused by an interactive effect of genetic and
environmental factors within a limited age-window. While both the
susceptibility genes and the candidate environmental exposures appear to be
quite common, the disease is still uncommon, raising a possibility of low
penetrance (253).
In mice, the host's genes restrict the diabetogenic effect of picornaviruses in
a manner compatible with a recessive trait not related to the MHC. In humans,
on the other hand, susceptibility to diabetogenic enteroviruses appears to be
genetically restricted by HLA-DR and DQ alleles (172). However, the allelic
specificity is controversial (55,254,255,256) and may depend on the viral type
and epidemicity. In general, the HLA-DR3 allele, present in most patients with
type 1 diabetes, is associated with viral persistence.
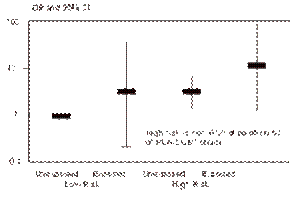
Figure 9.6. Odds ratios for type 1
diabetes by exposure to whole cow’s milk prior to 3 months of age in low- and
high- risk individuals, as determined by a genetic marker on the HLA-DQB1 chain
(255).
Very few studies have examined a possibility
of an interaction between the HLA genes and dietary exposures (255,230)
(see Fig. 9.6). The epidemiological data are limited, but suggest that an early
exposure to cow's milk in relatives with HLA-DR3/4, DQB1*0302, DR3/3 or DRx/4, DQB1*0302
is not associated with development of ß-cell autoantibodies (257,258).
It is unclear whether other genes are involved.
Remission ("Honeymoon
Period"): Shortly after clinical onset, most of the
patients experience a transient fall in insulin requirements due to improving ß-cell
function. Total and partial remissions have been reported in, respectively,
2-12% and 18-62% of young type 1 diabetes patients (54,259,260). Older age and
less severe initial presentation of diabetes (259,260,261,262)
and low or absent ICA (262,263,264)
or IA-2 (265)
have been consistently associated with deeper and longer remission (5).
Evidence relating GAD autoantibodies (262,265,266),
non-Caucasian origin, HLA-DR3 allele, female gender and family history of type
1 diabetes to a less severe presentation, greater frequency of remission and
slower deterioration of insulin secretion is inconclusive. Most studies (259,263),
but not all (260,261), agree that preserved ß-cell function is associated with
better glycemic control (lower HbA1c) and preserved ß-cell glucagon response to
hypoglycemia (267). The prevalence of ICA (but not GAA) decreases from 87% at
the time of type 1 diabetes diagnosis to 38-62% 2-3 years later (263,268),
faster in young boys, subjects lacking HLA-DR3 and 4, and those diagnosed
between July and December (268).
The natural remission is always temporary, ending with a gradual or abrupt
increase in exogenous insulin requirements. Destruction of ß-cells is complete
within 3 years of diagnosis in most young children, especially those with the
HLA DR3/4 genotype (218). It is much slower and often only partial in older
patients (270), 15% of who still have some ß-cell function preserved 10 years
after diagnosis (271).
Established Diabetes
Acute complications: Acute complications of type 1 diabetes: DKA,
hypoglycemia and infections are described in detail in other chapters. The risk
of hospital admission for acute complication is 30/100 patient-years (p-yrs) in
the first year of the disease and 20/100 p-yrs in the subsequent 3 years (54).
Age and sex-specific incidence pattern suggest that the risk of ketoacidosis is
increased in adolescent girls (272).
The preventable or potentially modifiable risk factors comprise lack of health
insurance, high HbA1C levels and mental problems (272,273).
An estimated 26% of the patients have at least one episode of severe
hypoglycemia within the initial 4 years of diagnosis, with little relation to
the demographic or socioeconomical factors. The incidence of severe
hypoglycemic episodes varies between 6 and 20 per 100 person-years, depending
on age, geographic location, and intensity of insulin treatment (54,272).
Mortality: Insulin treatment dramatically prolongs survival
but it does not cure diabetes. Although the absolute mortality at onset and
within the first 20 years of type 1 diabetes is low (3-8%), it is 5 times
higher for diabetic males and 12 times higher for diabetic females, compared to
the general population (274,275).
In the
Cardiovascular disease and renal disease are the most common causes of death of
type 1 diabetic subjects accounting for 44% and 21% respectively (278).
Analyses of mortality from the cohort of patients with type 1 diabetes have
also shown that cerebrovascular mortality (stroke of nonhemorrhagic origin) is
raised at all ages in these patients (279).
The risk of mortality from ischaemic heart disease is exceptionally high in
young adult women, with rates similar to those in men under the age of 40 with
type 1 diabetes (280,281).
The excess mortality is lowest in Scandinavia, intermediate in the
Moreover WHO Multinational Study of Vascular Disease in Diabetes performed in
10 centers throughout the word from 1975 to 1987 showed that age-adjusted all
cause mortality rates were significantly higher in type 1 diabetes compared
with type 2 diabetes.
Survival and avoidance of complications have been related to better metabolic
control (285),
but genetic factors also appear to be involved. In the EURODIAB study the
predictors for cardiovascular mortality were blood pressure, serum cholesterol,
proteinuria and retinopathy, but not fasting plasma glucose (286). In line with
this observation, insulin resistance-related factors, but not glycemia,
predicted coronary artery disease in type 1 diabetes during 10-year follow-up
in the Pittsburgh Epidemiology of Diabetes Complications study (287).
The strongest predictors for five-year mortality in patients with type 1 are
amputations (hazard ratio, HR=5.08) and poor visual acuity (HR=1.74) (275).
Inconsistent associations have been reported between diabetic nephropathy and
HLA-DR4 (288)
and several genes involved in blood pressure regulation (285,289,290,291,292,293,294,295).
Polymorphisms of paraoxonase (296,297)
and A-IV (298) appear to play an important role in development of coronary
artery disease in type 2 diabetes patients, but have not been extensively
studied in persons with type 1 diabetes.
Microvascular complications:
The high incidence, associated severe morbidity (299), mortality (300),
and enormous health care expenditures(301,302,303)
make T1DM a prime target for interventions.
The Diabetes Control and Complications Trial demonstrated the efficacy of tight
glycemic control in reducing the risk of late complications of type 1 diabetes
(304,305,306).
Unfortunately, increased risk of hypoglycemia associated with tight control
(307,308)
and compliance problems have hampered full implementation of the trial
recommendations, especially in children. Importantly, studies, including ours,
have shown that factors other than hyperglycemia contribute significantly to
development of microvascular complications.
Diabetic retinopathy:
Diabetes is the most common cause of new cases of blindness in the
Alterations in retinal blood flow and loss of retinal pericytes (310)
precede the earliest clinical stages of diabetic retinopathy. In contrast to
the loss of retinal pericytes, changes in retinal arterial blood flow can be
detected non-invasively using laser Doppler velocimetry (311),
video fluorescein angiography (312)
or pulsatile ocular blood flow (313).
T1 DM patients with no retinopathy or mild NPDR show dilated major retinal
arteries with reduced blood flow velocity, compared to that in non-diabetic
controls (312,314).
With increasing duration of diabetes and progression of retinopathy, retinal
blood flow shows a bimodal pattern of a transition from reduced to increased (311,315).
Diabetic nephropathy:
Historically, diabetic nephropathy would affect 30-50% of T1DM patients
throughout the initial 20 years of the disease (316,317,318). While there has
been an evidence for a secular decline in the incidence of nephropathy in T1DM
(317,319,320), this decline may have leveled off (321)
and the cumulative risk remains at least 20%-30%.
The incidence peaks 10-15 years after diagnosis
and then declines, suggesting that only a subset of the patients is susceptible
to diabetic nephropathy. Increased urinary albumin excretion or
microalbuminuria (MA) has been shown to be an early manifestation of diabetic
nephropathy, rather than merely a prognostic factor (322,323,324).
Since MA strongly predicts progression to later stages of diabetic nephropathy
(overt proteinuria and end-stage renal disease) (325,326), current standards of
care include screening for MA and treatment of those positive for MA with ACE
inhibitors (327,328,329,330).
However, prediction of the progression to overt nephropathy based on the
presence of MA and the other risk factors is imprecise. In addition, some
patients in whom MA has not increased may nonetheless develop advanced renal
lesions (331).
Previously, increased GFR (332,333),
increased ambulatory blood pressure (334,335),
and autonomic neuropathy (335,336)
have shown some promise but have not gained wide acceptance.
Recent reports from patients with type 1 and type 2 diabetes suggest that
podocyte loss is associated with progression of diabetic glomerulosclerosis (337,338,339,340,341).
Diabetic neuropathy: The
incidence of neuropathy in EURODIAB study group was independently associated
with duration of diabetes, glycosylated hemoglobin values, BMI and smoking (342).
Risk factors for microvascular complications
in type 1 diabetes: Traditional epidemiological distinction
between micro- and macrovascular complications of diabetes is becoming more
fluid with the recognition that similar processes (e.g., glycation,
hypertension, inflammation, and endothelial dysfunction) affect both vascular
beds. Accurate markers of early retinal microvascular alterations may offer a
powerful predictive tool concerning the risk of cumulative vascular burden and
future micro- and macrovascular clinical events elsewhere in the body.
Hyperglycemia: Chronic
hyperglycemia is probably the most accepted risk factor for development of
microvascular complications in type 1 diabetes mellitus (304,343,344,345,346).
Blood pressure: Numerous
previous studies have demonstrated that increased systolic or diastolic blood
pressure is a powerful predicator of microvascular complications (347,348,349,350).
Cigarette smoking is an
established risk factor for diabetic nephropathy (348,351,352).
Interestingly, smoking does not appear to be an independent risk factor for
diabetic retinopathy (353).
Lipids and lipoproteins: Elevated
TG and low HDL-cholesterol (349,354,355),
and increased total and LDL-cholesterol (348,356,357)
have been reported to predict diabetic retinopathy and diabetic nephropathy.
Insulin resistance is
an emergent risk factors for both macro- and microvascular complications in
type 1 DM (354,355).
However, it is unclear if this phenomenon is mediated by associated risk
factors (350)
or a common genetic determinant (358,359).
Visceral obesity, PAI-1 and markers of
inflammation and endothelial dysfunction tend to cluster with
microalbuminuria and insulin resistance in diabetic and nondiabetic persons and
are clearly associated with macrovascular complications. The relationship of
these markers to microvascular diabetic complications has been less studied (360,361).
Visceral obesity (354,355),
vWF and fibrynogen (362,349),
C-reactive protein, IL-6, TNF-a (361)
as well as PAI-1 levels (363)
have been found to predict diabetic nephropathy and/or diabetic retinopathy.
Most of the previous studies were cross-sectional and none has measured
visceral obesity directly or analyzed all of these factors jointly. This
prospective study will be in a unique position to shed new light on this
cluster of novel microvascular risk factors.
Age at onset of diabetes, C-peptide:
Greater severity of initial presentation, related largely to the HLA-DR/DQ
genotype and age at diagnosis, predicts the faster loss of endogenous insulin
production and poorer glycemic control. Lower endogenous insulin production
(marked by undetectable C-peptide levels and associated with higher HbA1c) has
been suggested to predict progression of diabetic retinopathy (364)
and diabetic nephropathy (365). These observations are consistent with the
association of microvascular complications with HLA-DR3/4 DR3/4 genotype
(366,367) and faster loss of GAD autoantibodies (368).
Importantly, it has been recently found by DAISY study that early diagnosis of
type 1 diabetes, in the group of children followed from prediabetic stage to
diabetes onset, is associated with better preservation of insulin secretion,
which resulted in lower mean insulin dose 12 months after diagnosis (369).
The significance of residual insulin secretion with regard to metabolic control
and to long-term complications was confirmed by data from DCCT study (370).
Patients with preserved C-peptide and lower insulin requirements, fasting blood
glucose and HbA1c in the intensive therapy group had 50% reduced risk for
progression of retinopathy, development of microalbuminuria and 65% lower risk
of severe hypoglycemia (370).
Genetics of microvascular complications:
Despite the seemingly inevitable development of complications, at least half of
the patients survive over 40 years and a quarter of these have no major
complications (371). Retinopathy develops in virtually all patients, given
enough time, but the more severe proliferative retinopathy and visual
impairment appear in only up to half of the patients (371). Survival and
avoidance of complications is related to better metabolic control, but patients
developing microvascular complications, especially nephropathy, frequently have
no identifiable classical risk factors, suggesting that the genetic component
of renal disease is different from that of diabetes.
Diabetic nephropathy clearly clusters in certain families, apparently
independent of glycemic control and both in type 1 (372,373,374)
and type 2 diabetes (375,376,377,378,379,380).
Familial clustering of diabetic nephropathy in T1DM families has been confirmed
in a study incorporating the results of kidney biopsies in addition to more
common measures of renal function such as urinary AER (381).
The clustering is more pronounced in African Americans than Caucasians (376,378,382).
In segregation analysis of the adenine/creatinine ratio as a continuous trait
in type 1 diabetes, adjusting for age, sex, and duration of diabetes,
adenine/creatinine inheritance was most consistent with a Mendelian model with
multifactorial inheritance (383),
i.e. determined by a mixture of genes, variable effects, and environment
factors such as diabetes and hypertension. Adenine/creatinine ratio
heritability (h2) was estimated to be 0.27.
Inconsistent associations have been reported between diabetic nephropathy and
HLA-DR4 (384)
and several genes involved in blood pressure regulation (371,385,386,387,388,389).
ApoE genotype may mediate well-known association between dyslipidemia and
development of diabetic nephropathy (390,391,392).
Matrix metalloproteinase-9 polymorphism may play a role (393).
Additional genes (IL1RN, NHE5, NOS1, KLKB1, RAGE,) may be minor contributors to
genetic risk. Extensive analysis of the chromosome 3 region suggested strong
evidence of a nephropathy gene, but a detailed evaluation of the AGTR1 gene
suggested that alleles of AGTR1 were not the source of the linkage.
The genetic components of retinopathy and other microvascular complications
have been investigated to a lesser degree than nephropathy. Diabetic
retinopathy clusters in families (307). Diabetic retinopathy has been
associated with the presence of genetic variants of the aldose reductase
promoter region, eNOS4 polymorphism of the endothelial nitric oxide synthase
and DNA sequence variants of VEGF and vitamin D receptor genes (394,395,396,397,398,399,400,
401).
Data concerning the role of HLA genotypes are inconsistent (366,367,402,403,404,405)
. The associations between diabetic nephropathy, diabetic retinopathy, and
markers of insulin resistance may be mediated by a polymorphism in the PC-1
gene coding region (358,359).
Macrovascular complications
Coronary artery disease (CAD):
CAD is the main cause of death in persons with type 1
diabetes and accounts for a large proportion of premature morbidity and
mortality in the general population. Heart disease in type 1 diabetic patients
occurs earlier in life, affects women as often as men, and associated mortality
is dramatically higher than that in the general population (406,407,408,409).
Women with type 1 diabetes are 9 to 29 times more likely to die of CAD than
nondiabetic women; the risk for men is increased 4 to 9-fold. Patients with proteinuria
are at a 15-37 times increased risk of fatal CAD while the risk of those
without proteinuria is 3-4-fold, compared to the general population (406,410).
However, it is far from clear whether the association of CAD with nephropathy
is mediated by hypertension and dyslipidemia - features of renal failure - or
rather by risk factors that predispose to both nephropathy and CAD. While
conventional CAD risk factors (hypertension, smoking, low HDL cholesterol and
high triglycerides) increase the risk, the role of hyperglycemia, autonomic
neuropathy, endothelial dysfunction, insulin resistance and diabetes duration
is less established (411,412,413,414).
The Pittsburgh Epidemiology of Diabetes Complications Study has recently
suggested that, among endothelial dysfunction markers, E-selectin concentration
is an independent predictor of CAD (415,416).
In type 1 diabetic patients, atherosclerosis is more diffuse (417,418), leading
to higher case fatality (419,420),
higher cardiac failure (421) and restenosis rates (422),
and shorter survival (422,423),
compared to the general population. These poor outcomes emphasize the need for
primary prevention of CAD in type 1 diabetic patients. Silent ischemia is
common - 24% of asymptomatic patients older than 35 yrs had ischemia on
exercise test, Holter monitoring or dynamic thallium scintigraphy and 10% had
coronary stenosis greater than 50% by angiography (424).
In asymptomatic young subjects with type 1 diabetes the incidence of CAD is
increasing 1-2% per year and by their mid 40s 70% of men and 50% of women
develop CAC (Coronary Artery Calcification) - a marker of atherosclerotic
plaque (425,426).
Small clinical studies using B-mode imaging of carotid arteries have suggested
that type 1 diabetic patients have significant atherosclerosis as early as at
the age of 10-19 yrs and strongly associated with diabetes duration (427,428).
This observation was recently confirmed by EDIC study (429).
After six years of follow-up the progression of the intima-media thickness of
the common carotid artery was significantly greater in patients with type 1
diabetes in comparison to the controls and was associated with age, mean HbA1C,
LDL/HDL ratio, smoking, systolic blood pressure and urinary albumin excretion rate
(429).
However, it is still little known about the risk factors for progression of
asymptomatic coronary artery disease to clinical endpoints. To address this
issue, 6 years ago, the study Coronary Artery Calcification (CAC) in Type 1
Diabetes (CACTI), was initiated (430,431,432,433,434,435,436,437).
The measurement of CAC by electron beam tomography is one of the new techniques
(besides the ultrasound imaging of carotid arteries and potentially coronary
magnetic resonance imaging) for noninvasive monitoring of cardiovascular
disease progression (438,439).
CAC has been shown to correlate well with the invasive method of coronary lumen
estimation (angiography) and arterial wall intimal architecture (IVUS) (435,438).
The initial data from the CACTI study suggest that gender-related differences
in insulin sensitivity may explain the increase in CAC in women compared with
men with type 1 diabetes (431).
Moreover a common promoter polymorphism in the hepatic lipase gene (LIPC-480C>T)
was found to be associated with the extent of coronary calcification in a dose
dependent manner (431).
Among type 1 diabetic patients greater progression of CAC was observed in
subjects with HbA1C values >7.5%, higher levels of soluble IL-2 receptor and
low plasma adiponectin (416,430,433)
One third of the patients enr
Summary
Type 1 diabetes can be diagnosed at any age, but clinical course, genetic, and
environmental determinants appear to be heterogenous by age. The common pathway
begins with pre-clinical ß-cell autoimmunity with progressive defect of insulin
secretion, followed by onset of hyperglycemia, transient usually partial
remission, and finally complete insulinopenia associated with acute and chronic
complications and premature death. Current research effort is focused on
identification of the genetic and environmental determinants of this process
and the ways they interact.
|
Location |
Year of Study |
Age (y) |
Prevalence |
|
|
|||
|
SEARCH study: Non-Hispanic White African American Hispanic Asian/Pacific Islander American Indian/Alaska Native All Race/Ethnicity |
2002 |
0-19 |
220 190 130 80 120 180 |
|
|
1999 |
0-19 |
180 |
|
American Indians |
1999-2001 |
0-20 |
70 |
|
|
1978-79 |
0-17 |
208 |
|
|
1970 |
0-14 |
57 |
|
|
1961 |
0-15 |
61 |
|
|
1993 |
0-14 |
120.4 |
|
|
|
|
|
|
|
1997 |
0-18 |
366 |
|
|
1999 |
0-21 |
158 |
|
|
1999 |
0-16 |
210 |
|
|
1998 |
total |
280
(F), 400 (M) |
|
|
1984-85 |
0-14 |
99 |
|
|
2003 |
0-14 |
101 |
|
|
1993-95 |
0-19 |
140.2 |
|
|
1973 |
0-14 |
83 |
|
|
1979 |
0-14 |
191 |
|
|
1977 |
0-14 |
300 |
|
|
1988 |
0-14 |
60 |
|
|
|||
|
|
1986 |
0-19 |
105 |
|
2001 |
0-20 |
227 |
|
|
|
1996 |
0-14 |
0.28 |
|
|
1984 |
0-14 |
57 |
|
|
2002 |
|
269 |
*
All types of diabetes
Table 9.1. Prevalence of childhood type
1 diabetes, from selected studies.
|
Country |
Region /ethnicity |
Time
of study |
Age |
Incidence Cases/
100.000 |
95%
CI |
Time
trend %/yr |
Ref. |
|
|
6 centres |
2002 |
0-9 |
|
NA |
- |
472 |
|
Non-Hispan.White |
21.6 |
||||||
|
African American |
11.8 |
||||||
|
Hispanic |
13.1 |
||||||
|
Asian/Pacific |
6.0 |
||||||
|
American Indian |
3.9 |
||||||
|
6 centres |
2002 |
9-19 |
|
NA |
- |
472 |
|
|
Non-Hispan.White |
22.5 |
||||||
|
African American |
19.3 |
||||||
|
Hispanic |
16.1 |
||||||
|
Asian/Pacific |
10.4 |
||||||
|
American Indian |
9.4 |
||||||
|
American Indians |
1999-2001 |
0-19 |
5.8 |
NA |
- |
||
|
|
1990-1994 |
0-14 |
|
|
NA |
||
|
Non-Hispan.White |
13.1 |
10.3-15.7 |
|||||
|
Hispanic |
15.4 |
9.5-23.5 |
|||||
|
African American: |
12.9 |
10.1-15.2 |
|||||
|
|
1985-1994 |
0-17 |
|
|
NA |
||
|
African American |
15.2 |
13.5-17.0 |
|||||
|
Latinos |
10.7 |
9.1-12.6 |
|||||
|
|
|
1987-2002 2002 |
0-14 |
35.9 47.6 |
31.8-40.0 |
1.4 |
|
|
|
|||||||
|
|
|
1986-2003 |
0-14 |
4.0 |
3.0-4.8 |
NA |
|
|
|
|
|
|
|
|
|
|
|
Country |
Region /ethnicity |
Time
of study |
Age |
Incidence Cases/
100.000 |
95%
CI |
Time
trend %/yr |
Ref. |
|
|
|
1990-1998 |
0-14 |
3.09 |
2.0-4.1 |
N.S. |
|
|
|
Nationwide |
1988-1997 |
0-14 |
3.94 |
NA |
7.6 |
|
|
|
|
1976-1999 |
0-14 |
5.7 |
4.5-7.0 |
8.9 |
|
|
|
Nationwide |
1982-1998 |
0-14 |
7.0 |
6.5-7.5 |
|
|
|
|
|
1990-2001 |
0-14 |
7.2 |
5.4-9.4 |
3.9 |
|
|
|
Nationwide |
1991-1998 |
0-14 |
M:6.7 |
5.7-7.9 |
N.S. |
|
|
F:7.2 |
6.1-8.4 |
||||||
|
|
Nationwide study |
1983-2000 |
0-14 |
7.5 |
7.1-8.0 |
2.3 |
|
|
|
|
1990-1999 |
0-14 |
7.4 |
3.5-11.3 |
NA |
|
|
|
Nationwide |
1978-1998 |
0-14 |
7.9 |
7.5-8.2 |
I |
|
|
|
Nationwide |
1990-1998 |
0-14 |
8.5 |
7.5-9.5 |
3.6 |
|
|
|
|
1988-1997 |
0-20 |
9.6 |
8.6-10.5 |
2.9 |
|
|
|
Nationwide |
1991-1999 |
0-14 |
7.8-10.6 |
NA |
5.1 |
|
|
|
Nationwide |
1989-1999 |
0-14 |
5.5-13.0 |
NA |
2.1 |
|
|
|
|
1989-1997 |
0-14 |
6.65 |
6.09-7.24 |
12.9 |
|
|
|
1988-1999 |
0-14 |
7.3 |
4.6-10.1 |
7.6 |
||
|
Three cities study |
1987-1999 |
0-14 |
8.4 |
7.4-9.3 |
I |
||
|
|
1997-2002 |
0-14 |
13.6 |
NA |
10,4 |
||
|
|
|
1989-2000 |
0-14 |
11.8 |
10.3-13.4 |
1.8 |
|
|
|
Nationwide |
1989 |
0-15 |
12.0 |
11.6-12.4 |
6.8 |
|
|
|
Nationwide |
1991-1998 |
0-14 |
M:12.5 |
10.7-14.6 |
2.7 |
|
|
F:10.9 |
9.1-12.8 |
1.5 |
|||||
|
|
North Rine-Westphalia |
1987-2000 |
0-14 |
13.1 |
12.1-14.1 |
3.6 |
|
|
|
Nationwide |
1985-2000 |
0-14 |
5.6-14.5 |
NA |
9.8 |
|
|
|
Nine centers |
1990-1999 |
0-14 |
|
|
|
|
|
Northern part |
12.2 |
10.3-12.2 |
2.1 |
||||
|
Central part |
9.3 |
8.8-9.9 |
3.6 |
||||
|
Southern part |
6.2 |
5.8-6.7 |
4.7 |
||||
|
( |
1989-1998 |
0-14 |
12.56 |
11.0-14.3 |
0.8 |
||
|
|
1984-2000 |
0-14 |
M:
10.7 F: 9.8 |
9.5-12.0 8.6-11.1 |
NA |
||
|
|
Nationwide |
1996-1999 |
0-14 |
18.6 |
17.7-19.4 |
3.2 |
|
|
|
|
1978-2000 |
0-15 |
15.5 |
14.9-16.1 |
2.9 |
|
|
Devon, |
1975-2001 |
0-14 |
16,08 |
14.8-17.3 |
3.0 |
||
|
|
1978-1998 |
0-14 |
|
|
|
||
|
South Asian origin |
13.0 |
9.9-16.2 |
6.5 |
||||
|
Caucasian origin |
12.9 |
11.2-14.6 |
2.8 |
||||
|
Leicestershire |
1989-1998 |
0-14 |
|
|
NA |
||
|
South Asian origin |
M:20.4 F:19.0 |
13.0-30.4 11.6-29.4 |
|||||
|
Caucasian origin |
M:17.7 F:17.7 |
14.8-20.9 14.8-21.1 |
|||||
|
|
|
2001-2002 |
0-14 |
17.6 |
NA |
NA |
|
|
|
1982-2000 |
0-14 |
16.3 |
15.1-17.4 |
3.8 |
||
|
|
1988-1998 |
0-14 |
16.8 |
14.1-19.8 |
NA |
||
|
|
Nationwide |
1996-2000 |
0-14 |
19.5 |
NA |
1.8 |
|
|
|
Nationwide |
1989-1998 |
0-15 |
22.4 |
21.5-23.5 |
N.S. |
|
|
|
Nationwide |
1983-2000 |
0-14 |
28.9 |
28.2-29.5 |
2.2 |
|
|
South-eastern part |
1977-2001 |
0-16 |
22.4-26.4 |
NA |
NA |
||
|
|
Nationwide |
1990-1999 |
0-14 |
41.4 |
37.3-45.5 |
I |
492, |
|
|
|
1989-
1999 1999 |
0-14 |
38.8 49.3 |
36.7-41.1 41.3-59.0 |
2.8 |
|
|
Nationwide |
1996-2000 |
0-14 |
0.08 |
N.A. |
|
|
|
|
Western part |
1985-2002 |
0-14 |
16.5 |
14.7-18.2 |
3.1 |
|
|
|
|
1970-1989 |
0-19 |
22.8 |
NA |
5.0 |
|
|
Nationwide |
1999-2000 |
0-14 |
17.9 |
15.9-20.0 |
NA |
||
|
|
|||||||
|
|
Northern part |
1991-1997 |
0-14 |
0.37 |
0.29-0.56 |
N.S. |
|
|
|
|
1997-2000 |
0-14 |
1.0 |
0.76-1.2 |
N.S. |
|
|
|
Nationwide |
1998 |
0-12 |
2.46 |
NA |
N.S. |
|
|
|
|||||||
|
|
Nationwide |
1991-1996 |
0-14 |
M:3.1 F:4.4 |
2.6-3.7 3.7-5.0 |
NA |
|
|
|
|
1991-2000 |
0-14 |
7.8 |
6.9-8.8 |
N.S. |
|
|
|
Nationwide |
1998 |
0-17 |
|
NA |
NA |
|
|
Jews origin |
9.5 |
||||||
|
Arab origin |
8.0 |
||||||
|
|
Nationwide |
1992-1997 |
0-14 |
20.1 |
18.0-22.1 |
I |
|
|
|
Eastern Province |
1986-1997 |
0-15 |
12.3 |
NA |
NA |
|
N.S.=not
significant increase or no increase NA= no available data, I-increase
Table 9.2. Current incidence of type 1 diabetes (data published 2000-2005).
References - Chapter 9
1. Rewers M. The changing face of the
epidemiology of insulin-dependent diabetes mellitus (IDDM): Research designs
and models of disease causation. Ann Med 23:419-426, 1991.
2. Libman I, Songer T, LaPorte R. How many
people in the
3. Rewers M, LaPorte RE, King H, Tuomilehto J.
Trends in the prevalence and incidence of diabetes: insulin-dependent diabetes
mellitus in childhood. World Health Stat Q 41:179- 189, 1988.
4. Patrick SL, Moy CS, LaPorte RE. The world of
insulin-dependent diabetes mellitus: what international epidemiologic studies
reveal about the etiology and natural history of IDDM. Diabetes Metab Rev
5:571-578, 1989.
5. Chase HP, MacKenzie TA, Burdick J, Fiallo-Scharer
R, Walravens P, Klingensmith G, Rewers M. Redefining the clinical remission
period in children with type 1 diabetes. Pediatr Diabetes. 2004 Mar;5(1):16-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15043685&query_hl=9
6. Songer TJ. Health services and costing in
diabetes. Med J Aust 152:115-117 , 1990.
7.
8. Skyler JS, Crofford OB, Dupre J, Eisenbarth GS,
Fathman GC, Gale EAM, Goldstein D, Harmon JT, Haymond MW, Jackson RA, Kahn R,
Krischer J, Maclaren NK, Palmer JP, Silverman R, Simon R. Prevention of
Type 1 diabetes mellitus. Diabetes 39:1151-1152, 1990.
9. American Diabetes Association. Report of the
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.
Diabetes Care 20:1183-1197, 1997.
10. Alberti KG, Zimmet PZ. Definition,
diagnosis and classification of diabetes mellitus and its complications. Part
1: diagnosis and classification of diabetes mellitus provisional report of a
WHO consultation [see comments]. Diabet Med 15:539-553, 1998. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9686693&query_hl=28
11. Atkinson MA, Eisenbarth GS. Type 1
Diabetes: New Perspectives on Disease Pathogenesis and Treatment. Lancet
2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11476858&query_hl=31
12. Maugendre D, Chaillous L, Rohmer V, Lecomte P,
Marechaud R, Sai P, Marre M, Charbonnel B, Allannic H, Delamaire M.
Multiple antibody status in type 1 diabetic patients and subjects at various
risk with islet-cell antibodies. Diabetes Metab 23:320-326, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9342546&query_hl=33
13. Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa
Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid
onset and an absence of diabetes-related antibodies. Osaka IDDM Study
Group. New Engl J Med 342:301-307, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10655528&query_hl=37
14. Rewers M, Norris J, Dabelea D. Epidemiology of
type 1 Diabetes Mellitus. Adv Exp Med Biol. 2004;552:219-46. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15622966&query_hl=39
15. Bottazzo GF, Florin-Christensen A, Doniach
D. Islet-cell antibodies in diabetes mellitus with autoimmune
polyendocrine deficiencies. Lancet 2:1279-1283, 1974.
16. Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati
O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent
diabetics before insulin treatment. Science 222:1337-1339, 1983.
17. Vardi P, Dib SA, Tuttleman M, Connelly JE,
Grinbergs M, Radizabeh A, Riley WJ, Maclaren NK, Eisenbarth GS, Soeldner
JS. Competitive insulin autoantibody assay. Prospective evaluation of
subjects at high risk for development of type I diabetes mellitus.
Diabetes 36:1286-1291, 1987.
18. Williams AJK, Bingley PJ, Bonifacio E, Palmer JP,
Gale EAM. A novel micro-assay for insulin autoantibodies. J
Autoimmun 10:473-478, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9376075&query_hl=42
19. Baekkeskov S, Nielsen JH, Marner B, Bilde T,
Ludvigsson J, Lernmark Å. Autoantibodies in newly diagnosed diabetic
children immunoprecipitate specific human pancreatic islet cell proteins.
Nature 298:167- 169, 1982.
20. Baekkeskov S, Aanstoot H-J, Christgau S, Reetz A,
Solimena M, Cascalho M, Folli F, Richter-Olesen H, DeCamilli P.
Identification of the 64K autoantigen in insulin-dependent diabetes as the
GABA-synthesizing enzyme glutamic acid decarboxylase [published erratum appears
in Nature 1990 Oct 25;347(6295):782]. Nature 347:151-156, 1990.
21. Rabin DU, Pleasic SM, Shapiro JA, Yoo-Warren H,
Oles J, Hicks JM, Goldstein DE, Rae PM. Islet cell antigen 512 is a
diabetes-specific islet autoantigen related to protein tyrosine
phosphatases. J Immunol 152:3183-3188, 1994.
22. Verge CF, Gianani R,
23. Bingley PJ, Christie
MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, Bottazzo GF, Gale
EA. Combined analysis of
autoantibodies improves prediction of IDDM in islet cell antibody-positive
relatives. Diabetes 43:1304-1310, 1994.
24. Bingley PJ, Bonifacio E, Williams AJ, Genovese S,
Bottazzo GF, Gale, EA. Prediction of IDDM in the general population:
strategies based on combinations of autoantibody markers. Diabetes
46:1701-1710, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9356015&query_hl=46
25. Barker JM, Barriga
KJ, Yu L, Miao D, Erlich HA, Norris JM, Eisenbarth GS, Rewers M; Diabetes
Autoimmunity Study in the Young. Prediction
of autoantibody positivity and progression to type 1 diabetes: Diabetes
Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab. 2004 Aug;89(8):3896-902. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15292324&query_hl=48
26. Yu J, Yu L, Bugawan
TL, Erlich HA, Barriga K, Hoffman M, Rewers M, Eisenbarth GS. Transient antiislet autoantibodies: infrequent
occurrence and lack of association with "genetic" risk factors. J Clin Endocrinol Metab.
2000 Jul;85(7):2421-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10902788&query_hl=50
27. Stanley HM, Norris
JM, Barriga K, Hoffman M, Yu L, Miao D, Erlich HA, Eisenbarth GS, Rewers M;
Diabetes Autoimmunity Study in the Young (DAISY). Is presence of islet autoantibodies at birth
associated with development of persistent islet autoimmunity? The Diabetes
Autoimmunity Study in the Young (DAISY). Diabetes Care. 2004 Feb;27 (2):
497-502. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14747235&query_hl=52
28. Rewers M. Islet autoantibodies in cord blood:
maternal, fetal, or neither? Diabetes Metab Res Rev. 2002 Jan-Feb;18(1):2-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11921412&query_hl=54
29. Bonifacio E, Bingley PJ, Shattock M, Dean BM ,
Dunger D, Gale EAM, Bottazzo GF. Quantification of islet-cell antibodies
and prediction of insulin-dependent diabetes. Lancet 335:147-149,
1990.
30.
31. McCulloch DKPalmer JP. The
appropriate use of B-cell function testing in the preclinical period of Type 1
diabetes. Diabetic Med 8:800-804, 1991.
32. Eisenbarth GS, Moriyama H, Robles DT, Liu E, Yu
L, Babu S, Redondo M, Gottlieb P, Wegmann D, Rewers M. Insulin autoimmunity:
prediction/precipitation/ prevention type 1A diabetes. Autoimmun Rev. 2002
May;1(3):139-45. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12849007&query_hl=56
33. Miao D, Yu L, Tiberti C, Cuthbertson DD, Rewers
M, di Mario U, Eisenbarth GS, Dotta F. ICA512 (IA-2) epitope specific assays
distinguish transient from diabetes associated autoantibodies. J Autoimmun.
2002 Mar;18(2):191-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11908951&query_hl=58
34. Robles DT, Eisenbarth GS, Wang T, Erlich HA,
Bugawan TL, Babu SR, Barriga K, Norris JM, Hoffman M, Klingensmith G, Yu L,
Rewers M; Diabetes Autoimmunity Study in the Young. Millennium award recipient
contribution. Identification of children with early onset and high incidence of
anti-islet autoantibodies. Clin Immunol. 2002 Mar;102(3):217-24. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11890708&query_hl=60
35. Park YS, Kawasaki E, Kelemen K, Yu L, Schiller
MR, Rewers M, Mizuta M, Eisenbarth GS, Hutton JC. Humoral autoreactivity to an
alternatively spliced variant of ICA512/IA-2 in Type I diabetes. Diabetologia.
2000 Oct;43(10):1293-301. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11079748&query_hl=62
36. Schlosser M, Koczwara K, Kenk H, Strebelow M,
Rjasanowski I, Wassmuth R, Achenbach P, Ziegler AG, Bonifacio E. In
insulin-autoantibody-positive children from the general population, antibody
affinity identifies those at high and low risk. Diabetologia. 2005 Jul 12;
[Epub ahead of print] http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16010521&query_hl=64
37. Achenbach P, Koczwara K, Knopff A, Naserke H,
Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin
anticipate the autoimmune cascade that leads to type 1 diabetes.J Clin Invest.
2004, 114: 589-97 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15314696&query_hl=66
38. Achenbach P, Warncke K, Reiter J, Naserke HE,
Williams AJ, Bingley PJ, Bonifacio E, Ziegler AG. Stratification of type 1
diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004
Feb;53(2):384-92. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14747289&query_hl=68
39. Spinas GA, Matter L,
Wilkin T, Staffelbach O, Berger W. Islet-cell
and insulin autoantibodies in first-degree relatives of type I diabetics: a
5-year follow-up study in a Swiss population. Adv Exp Med Biol
246:209-214, 1988.
40. Thivolet C, Beaufrere B, Betuel H, Gebuhrer L,
Chatelain P, Durand A, Tourniaire J, Francois R. Islet cell and insulin
autoantibodies in subjects at high risk for development of type 1 (insulin-dependent)
diabetes mellitus: the
41. Maclaren N, Horne G, Spillar R, Barbour H,
Harrison D, Duncan J. The feasibility of using
42. Landin-Olsson M, Palmer JP, Lernmark A, Blom A,
Sundkvist G, Nystrom L, Dahlquist G. Predictive value of islet cell and
insulin autoantibodies for Type 1 (insulin-dependent) diabetes mellitus in a
population- based study of newly-diagnosed diabetic and matched control
children. Diabetologia 35:1068-1073, 1992.
43. Karjalainen JK. Islet cell antibodies as
predictive markers for IDDM in children with high background incidence of
disease. Diabetes 39:1144-1150, 1990.
44. Yu J, Yu L, Bugawan TL, Erlich HA, Barriga K,
Hoffman M, Rewers M, Eisenbarth GS. Transient anti-islet autoantibodies:
infrequent occurrence and lack of association with genetic risk factors. J Clin Endocrinol Metab
85:2421-2428, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10902788&query_hl=70
45. Lindberg BA,
Ericsson UB, Kockum I, Lernmark A, Landin-Olsson M, Sundkvist G, Ivarsson
SA. Prevalence of beta-cell and
thyroid autoantibody positivity in schoolchildren during three-year
follow-up. Autoimmunity 31 :175-185, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10739334&query_hl=72
46. Yu J, Yu L, Bugawan TL, Erlich HA, Barriga K,
Hoffman M, Rewers M, Eisenbarth GS. Transient anti-islet autoantibodies:
infrequent occurrence and lack of association with genetic risk factors. J Clin Endocrinol Metab
85:2421-2428, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10902788&query_hl=70
47. Riley WJ, Maclaren
NK, Krischer J, Spillar RP, Silverstein JH, Schatz DA , Schwartz S, Malone J,
Shah S, Vadheim C, Rotter JI. A
prospective study of the development of diabetes in relatives of patients with
insulin-dependent diabetes. N Engl J Med 323:1167-1172, 1990. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2215594&query_hl=74
48. Groop LC, Bottazzo
GF, Doniach D. Islet Cell
Antibodies Identify Latent Type I Diabetes in Patients Aged 35-75 Years at
Diagnosis. Diabetes 35:237-241, 1986.
49. Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles
W, Mackay IR. Antibodies to glutamic acid decarboxylase reveal
latent autoimmune diabetes mellitus in adults with a non-insulin- dependent
onset of disease. Diabetes 42:359-362, 1993.
50. Zimmet PZ, Tuomi T, Mackay IR, Rowley MJ, Knowles
W, Cohen M, Lang DA. Latent autoimmune diabetes mellitus in adults
(LADA): the role of antibodies to glutamic acid decarboxylase in diagnosis and
prediction of insulin dependency. Diabet Med 11:299-303, 1994.
51. Hamman RF, Cook M, Keefer S, Young WF, Finch JL,
Lezotte D, McLaren B, Orleans M, Klingensmith G, Chase HP. Medical care
patterns at the onset of insulin-dependent diabetes mellitus: association with
severity and subsequent complications. Diabetes Care 8 (Suppl
1):94-100, 1985.
52. Karjalajnen S, Salema P, IIonen J. A
comparison of childhood and adult type 1 diabetes mellitus. N Engl J Med
320:881- 886, 1989.
53. Levy-Marchal C, Papoz L, de Beaufort C, Doutreix
J, Froment V, Voirin J, Czernichow P. Clinical and laboratory features of
type I diabetic children at time of diagnosis. Diabetic Med
9:279-284, 1992.
54. Pinkney JH, Bingley PJ, Sawtell PA, Dunger DB,
Gale EAM, The Bart's-Oxford Study Group. Presentation and progress of
childhood diabetes mellitus: A prospective population-based study.
Diabetologia 37:70-74, 1994.
55. Eberhardt MS, Wagener DK, Orchard TJ, LaPorte RE,
Cavender DE, Rabin BS,
56. Foulis AK, Liddle CN, Farquharson MA,
57. Childhood Diabetes Research Committee MoHaWJ,
Polish Diabetes Research Group P, The Netherlands
Institute for Preventive Health Care L,
58. Kostraba JN, Gay EC, Rewers M, Chase HP,
Klingensmith GJ, Hamman RF. Increasing trend of outpatient management of
children with newly diagnosed IDDM. Colorado IDDM registry, 1978-88.
Diabetes Care 15:95- 100, 1992.
59. Rewers M, LaPorte RE, King H, Tuomilehto J.
Trends in the prevalence and incidence of diabetes: insulin-dependent diabetes
mellitus in childhood. World Health Stat Q 41:179- 189, 1988.
60. Libman I, Songer T, LaPorte R. How many
people in the
61. Diabetes Epidemiology Research International
Group. Geographic patterns of childhood insulin-dependent diabetes
mellitus. Diabetes 37:1113-1119, 1988.
62. Green A, Gale EAM, Patterson CC. Incidence
of childhood-onset insulin-dependent diabetes mellitus: the EURODIAB ACE
Study. Lancet 339:905-909, 1992.
63. Karvonen M, Viik-Kajander M, Moltchanova E,
Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes
worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care
23:1516-1526, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11023146&query_hl=76
64. Rewers MKostraba JN. Epidemiology of Type I
diabetes. 1994.
65. Adojaan B, Knip M, Vahasalo P, Karjalainen J ,
Kalits I, Akerblom HK. Relationship between the incidence of childhood
IDDM and the frequency of
66. Pilcher CC, Dickens K, Elliott RB.
67. Tajima N, LaPorte RE, Hibi I, Kitagawa T, Fujita
H, Drash AL. A comparison of the epidemiology of youth-onset
insulin- dependent diabetes mellitus between
68. Rewers M, Stone RA, LaPorte RE, Drash AL, Becker
DJ, Walczak M, Kuller LH. Poisson regression modeling of temporal
variation in incidence of childhood insulin-dependent diabetes mellitus in
69. Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford
D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent
diabetes mellitus among adolescents. Journal of Pediatrics 128:Part
1):608-615, 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8627431&query_hl=78
70. Rosenbloom AL, Joe JR, Young RS, Winter WE.
Emerging epidemic of type 2 diabetes in youth. Diabetes Care
22:345-354, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10333956&query_hl=80
71. Dabelea D, Hanson RL, Bennett PH, Roumain J ,
Knowler WC, Pettitt DJ. Increasing prevalence of Type II diabetes in
American Indian children. Diabetologia 41:904-910, 1998. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9726592&query_hl=82
72. Joner GSovik O. The incidence of type 1
(insulin-dependent) diabetes mellitus 15- 29 years in
73. Muntoni SSongini M. High incidence rate of
IDDM in
74. Bruno G, Merletti F,
Vuolo A, Pisu E, Giorio M, Pagano G. Sex differences in incidence of IDDM in age group 15-29 yr. Higher risk in males in
75. Christau B, Kromann H, Andersen OO, Christy M,
Buschard K, Arnung K, Kristensen IH, Peitersen B, Steinrud J, Nerup J.
Incidence, seasonal and geographic patterns of juvenile-onset insulin-dependent
diabetes mellitus is
76. Melton LJ, III, Palumbo PJ,
77. Turner R, Stratton I, Horton V, Manley S, Zimmet
P, Mackay IR, Shattock M, Bottazzo GF, Holman R. UKPDS 25:
autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for
prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes
Study Group [published erratum appears in Lancet 1998 Jan
31;351(9099):376]. Lancet 350:1288-1293, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9357409&query_hl=84
78. Horton V, Stratton I, Bottazzo GF, Shattock M,
Mackay I, Zimmet P, Manley S, Holman R, Turner R. Genetic heterogeneity
of autoimmune diabetes: age of presentation in adults is influenced by HLA DRB1
and DQB1 genotypes (UKPDS 43).
79. Diabetes Epidemiology Research International
Group. Geographic patterns of childhood insulin-dependent diabetes
mellitus. Diabetes 37:1113-1119, 1988.
80. Kostraba JN, Gay EC, Cai Y, Cruickshanks KJ,
Rewers MJ, Klingensmith GJ, Chase HP, Hamman RF. Incidence of
insulin-dependent diabetes mellitus in
81. Dabelea D on behalf of SEARCH Study Group.The
SEARCH for diabetes in youth study group. Abstracts of
82. Rewers M, Zimmet P. The rising tide of childhood
type 1 diabetes--what is the elusive environmental trigger? Lancet. 2004 Nov
6-12;364(9446):1645-7. Prevalence of type 1 diabetes
83. Feltbower RG, Bodansky HJ, McKinney PA, et al.
Trends in the incidence of childhood diabetes in south Asians and other
children in Bradford, UK Diab Med 19 (2): 162-166 FEB 2002 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11874434&query_hl=89
84. Siemiatycki J, C
85. Zimmet PZ, Rowley
MJ, Mackay IR, Knowles WJ , Chen QY, Chapman LH, Serjeantson SW. The ethnic distribution of antibodies to glutamic
acid decarboxylase: presence and levels of insulin-dependent diabetes mellitus
in Europid and Asian subjects. J Diabetes Complications 7:1-7,
1993.
86. Gale EA, Gillespie KM. Diabetes and
gender. Diabetologia 44:3-15, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11206408&query_hl=91
87. Karvonen M, Pitkaniemi M, Pitkaniemi J, Kohtamaki
K, Tajima N, Tuomilehto J. Sex difference in the incidence of
insulin-dependent diabetes mellitus: an analysis of the recent epidemiological
data. World Health Organization DIAMOND Project Group. Diabetes Metab Rev
13:275-291, 1997.
88. Krischer JP, Cuthbertson DD, Greenbaum C;
Diabetes Prevention Trial-Type 1 Study Group. Male sex increases the risk of
autoimmunity but not type 1 diabetes. Diabetes Care. 2004 Aug;27(8):1985-90.
89. Serban V, Timar R, Dabelea D, Green A,
90. Karvonen M,
Pitkaniemi M, Pitkaniemi J, Kohtamaki K, Tajima N, Tuomilehto J. Sex difference in the incidence of insulin-dependent
diabetes mellitus: an analysis of the recent epidemiological data. World Health
Organization DIAMOND Project Group. Diabetes Metab Rev 13:275-291,
1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9509279&query_hl=95
91. Green A, Hauge M,
92. Bruno G, Merletti F,
Vuolo A, Pisu E, Giorio M, Pagano G. Sex differences in incidence of IDDM in age group 15-29 yr. Higher risk in males in
93. Ustvedt HJOlsen E. Incidence of diabetes
mellitus in
94. Roglic G, Pavlic-Renar I, Sestan-Crnek S, Prasek
M, Kadrnka-Lovrencic M, Radica A, Metelko Z. Incidence of IDDM during
1988-1992 in
95. Vandewalle CL, Coeckelberghs MI, De Leeuw IH, Du
Caju MV, Schuit FC, Pipeleers DG, Gorus FK. Epidemiology, clinical
aspects, and biology of IDDM patients under age 40 years. Comparison of data
from
96. Risch N, Ghosh S, Todd JA. Statistical
evaluation of multiple-locus linkage data in experimental species and its
relevance to human studies: application to nonobese diabetic (NOD) mouse and
human insulin- dependent diabetes mellitus (IDDM). Am J Hum Genet 53:702-714,
1993.
97. Gale EA, Gillespie
KM. Diabetes and gender. Diabetologia 44:3-15, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11206408&query_hl=102
99. Kostraba JN, Gay EC, Cai Y, Cruickshanks KJ,
Rewers MJ, Klingensmith GJ, Chase HP, Hamman RF. Incidence of
insulin-dependent diabetes mellitus in
100. Dahlquist G, Gustavsson KH, Holmgren G, Hagglof
B, Larsson Y, Nilsson KO, Samuelsson G, Sterky G, Thalme B, Wall S. The
incidence of diabetes mellitus in Swedish children 0-14 years of age. A
prospective study 1977-1980. Acta Paediatr Scand 71:7-14, 1982.
101. Weinberg CR, Dornan TL, Hansen JA,
102. Ludvigsson JAfoke AO. Seasonality of type
1 (insulin-dependent) diabetes mellitus: values of C-peptide, insulin
antibodies and haemoglobin A1c show evidence of a more
rapid loss of insulin secretion in epidemic patients. Diabetologia
32:84-91, 1989.
103. Rewers M, LaPorte R, Walczak M, Dmochowski K,
Bogaczynska E. Apparent epidemic of insulin-dependent diabetes mellitus
in midwestern
104. Diabetes Epidemiology Research International
Group. Secular trends in incidence of childhood IDDM in 10
countries. Diabetes 39:858-864, 1990.
105. Nystrom L, Dahlquist G, Rewers M, Wall S.
The Swedish childhood diabetes study: an analysis of the temporal variation in
diabetes incidence, 1978-1987. Int J Epidemiol 19:141-146, 1990.
106. Tuomilehto J, Rewers M, Reunanen A, Lounamaa P,
Lounamaa R, Tuomilehto-Wolf E, Akerblom HK. Increasing trend in type I
(insulin-dependent) diabetes mellitus in childhood in
107. Dokheel TM. An epidemic of childhood
diabetes in the
108. Variation and trends in incidence of childhood
diabetes in
109. Karvonen M, Pitkaniemi J, Tuomilehto J.
The onset age of type 1 diabetes in Finnish children has become younger. The
Finnish Childhood Diabetes Registry Group. Diabetes Care
22:1066-1070, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10388969&query_hl=105
110. Rosenbauer J,
Herzig P, von Kries R, Neu A, Giani G. Temporal, seasonal, and geographical incidence patterns of type I
diabetes mellitus in children under 5 years of age in
111. Cinek O, Lanska V,
Kolouskova S, Sumnik Z, Snajderova M, Ronningen KS, Vavrinec J. Type 1 diabetes mellitus in Czech children diagnosed
in 1990-1997: a significant increase in incidence and male predominance in the
age group 0-4 years. Collaborators of the Czech Childhood Diabetes
Registry. Diabetic Med 17:64-69, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10691162&query_hl=109
112.
113. Schoenle EJ, Lang-Muritano M, Gschwend S, Laimbacher
J, Mullis PE, Torresani T, Biason-Lauber A, Molinari L. Epidemiology of
type I diabetes mellitus in
114. Cordell HJ, Todd JA. Multifactorial
inheritance in type 1 diabetes. [Review]. Trends Genet 11:499-504,
1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8533167&query_hl=116
115. Rewers M, Stone RA, LaPorte RE, Drash AL, Becker
DJ, Walczak M, Kuller LH. Poisson regression modeling of temporal
variation in incidence of childhood insulin-dependent diabetes mellitus in
116. Tuomilehto J, Rewers M, Reunanen A, Lounamaa P,
Lounamaa R, Tuomilehto-Wolf E, Akerblom HK. Increasing trend in type 1
(insulin-dependent) diabetes mellitus in childhood in
117. Nystrom L, Dahlquist G, Rewers M, Wall S.
The Swedish childhood diabetes study. An analysis of the temporal variation in
diabetes incidence 1978-1987. Int J Epidemiol 19:141-146, 1990.
118. Bruno G, Merletti
F, Biggeri A, Cerutti F, Grosso N, De Salvia A, Vitali E, Pagano G. Increasing trend of type I diabetes in children and
young adults in the
119. Onkamo P, Vaananen
S, Karvonen M, Tuomilehto J. Worldwide
increase in incidence of Type I diabetes--the analysis of the data on published
incidence trends. Diabetologia 42:1395-1403, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10651256&query_hl=120
120. Stene LC, Barriga
K, Norris JM, Hoffman M, Klingensmith G, Erlich HA, Eisenbarth GS, Rewers
M. Symptoms of common maternal
infections in pregnancy and risk of islet autoimmunity in early childhood. Diabetes
Care. 2003 Nov;26(11):3136-41. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14578251&query_hl=122
121. Wagener DK, Sacks JM, LaPorte
RE, MacGregor JM. The
122. Allen C, Palta M, D'Alessio DJ. Risk of diabetes in siblings and other relatives of IDDM subjects.
Diabetes 40:831-836, 1991.
123. Redondo MJ, Rewers M, Yu L, Garg S, Pilcher CC,
Elliott RB, Eisenbarth GS. Genetic determination of islet cell
autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of
patients with type 1 diabetes: prospective twin study. BMJ. 1999 Mar
13;318(7185):698-702. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10074012&query_hl=124
124. Steck AK, Barriga
KJ, Emery LM, Fiallo-Scharer RV, Gottlieb PA, Rewers MJ. Secondary attack rate of type 1
diabetes in
125. Lorenzen T, Pociot
F, Hougaard P, Nerup J. Long-term risk of IDDM in first-degree relatives of patients with IDDM. Diabetologia 37:321-327,
1994.
126. O'Leary LA, Dorman JS, LaPorte RE, Orchard TJ,
Becker DJ, Kuller LH, Eberhardt MS, Cavender DE, Rabin BS, Drash AL.
Familial and sporadic insulin-dependent diabetes: Evidence for heterogeneous
etiologies? Diab Res Clin Pract 14:183-190, 1991.
127. Erlich HA,
128. Bertrams JBaur MP. Insulin-dependent
diabetes mellitus. 348-358, 1984.
129. Todd JA. The role of MHC
class II genes in susceptibility to insulin- dependent diabetes mellitus.
Curr Top Microbiol Immunol 164:17-40, 1990.
130. Ide A, Babu SR, Robles DT, Wang T, Erlich HA,
Bugawan TL, Rewers M, Fain PR, Eisenbarth GS. "Extended" A1,
B8, DR3 haplotype shows remarkable linkage disequilibrium but is similar to
nonextended haplotypes in terms of diabetes risk. Diabetes. 2005
Jun;54(6):1879-83. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15919812&query_hl=128
131. Cruz TD, Valdes AM,
Santiago A, Frazer de Llado T, Raffel LJ, Zeidler A, Rotter JI, Erlich HA,
Rewers M, Bugawan T, Noble JA. DPB1
alleles are associated with type 1 diabetes susceptibility in multiple ethnic
groups. Diabetes. 2004 Aug;53(8):2158-63.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15277401&query_hl=130
132. Rewers A, Babu S, Wang TB, Bugawan TL, Barriga
K, Eisenbarth GS, Erlich HA.Ethnic differences in the associations between the
HLA-DRB1*04 subtypes and type 1 diabetes.Ann N Y Acad Sci. 2003, 1005: 301-9 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14679080&query_hl=132
133. Rewers A, Babu S, Wang TB, Bugawan TL, Barriga
K, Eisenbarth GS, Erlich HA. Ethnic differences in the
associations between the HLA-DRB1*04 subtypes and type 1 diabetes. Ann N
Y Acad Sci. 2003 Nov;1005:301-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14679080&query_hl=134
134. Rewers M, Bugawan TL, Norris JM, Blair A, Beaty
B, Hoffman M, McDuffie RS, Hamman RF, Klingensmith G, Eisenbarth GS, Erlich
HA. Newborn screening for HLA markers associated with IDDM: Diabetes
Autoimmunity Study in the Young (DAISY). Diabetologia 39:807-812,
1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8817105&query_hl=135
135. Pugliese A, Gianani
R, Moromisato R, Awdeh ZL, Alper CA, Erlich HA, Jackson RA, Eisenbarth
GS. HLA-DQB1*0602 is associated
with dominant protection from diabetes even among islet cell antibody-positive
first-degree relatives of patients with IDDM. Diabetes 44:608-613, 1995.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7789622&query_hl=137
136. Barker JM,
Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, Eisenbarth GS, Rewers M;
Diabetes Autoimmunity Study in the Young. Prediction
of autoantibody positivity and progression to type 1 diabetes:
Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab. 2004
Aug;89(8):3896-902. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15292324&query_hl=139
137. Kukko M, Virtanen SM, Toivonen A, Simell S,
Korhonen S, Ilonen J, Simel O, Knip M. Geographical variation in risk HLA-DQB1
genotypes for type 1 diabetes and signs of beta-cell autoimmunity in a
high-incidence country. Diabetes Care. 2004 Mar;27(3):676-81 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14988284&query_hl=140
138. Knip M, Kukko M, Kulmala P, Veijola R, Simell O,
Akerblom HK, Ilonen J. Humoral beta-cell autoimmunity in relation to
HLA-defined disease susceptibility in preclinical and clinical type 1 diabetes.
Am J Med Genet. 2002 May 30;115(1):48-54.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12116176&query_hl=142
139. Greenbaum CJ, Eisenbarth G, Atkinson M, Yu L,
Babu S, Schatz D, Zeidler A, Orban T, Wasserfall C, Cuthbertson D, Krischer J;
DPT-1 study group. High frequency of abnormal glucose
tolerance in DQA1*0102/DQB1*0602 relatives identified as part of the Diabetes
Prevention Trial--Type 1 Diabetes.
140. Steck AK, Bugawan TL, Valdes AM, Emery LM, Blair
A, Norris JM, Redondo MJ, Babu SR, Erlich HA, Eisenbarth GS, Rewers MJ. Association of non-HLA genes with type 1 diabetes autoimmunity.
Diabetes. 2005 Aug;54(8):2482-6.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16046318&query_hl=144
141. Davies JL, Kawaguchi Y, Bennett ST, Copeman JB,
Cordell HJ, Pritchard LE, Reed PW, Gough SCL, Jenkins SC, Palmer SM, Balfour
KM, Rowe BR, Farrall M, Barnett AH, Bain SC, Todd JA. A
genome-wide search for human type 1 diabetes susceptibility genes.
Nature 371:130-136, 1994.
142. Hashimoto L, Habita C, Beressi JP, Delepine M,
Besse C, Cambon-Thomsen A , Deschamps I, Rotter JI, Djoulah S, James MR,
Froguel P, Weissenbach J, Lathrop GM, Julier C. Genetic
mapping of a susceptibility locus for insulin-dependent diabetes mellitus on
chromosome 11q. Nature 371:161-164, 1994.
143. Owerbach D, Gabbay KH. The HOXD8 locus
(2q31) is linked to type I diabetes: interaction with chromosome 6 and 11
disease susceptibility genes. Diabetes 44:132-136, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7813807&query_hl=147
144. Field LL, Tobias R, Magnus T. A locus on
chromosome 15q26(IDDM3) produces susceptibility to
insulin-dependent diabetes mellitus. Nat Genet 8:189-194, 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7842018&query_hl=149
145. Copeman JB, Cucca F, Hearne CM, Cornall RJ ,
Reed PW, Ronningen KS, Undlien DE, Nisticò L, Buzzetti R, Tosi R, Pociot F,
Nerup J, Cornélis F, Barnett AH, Bain SC, Todd JA. Linkage
disequilibrium mapping of type 1 diabetes susceptibility gene (IDDM7) to
chromosome 2q31-q33. Nat Genet 9:80-85,
1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7704030&query_hl=151
146. Cox NJ, Wapelhorst B, Morrison VA, Johnson L,
Pinchuk L, Spielman RS, Todd JA, Concannon P. Seven regions of the genome
show evidence of linkage to type 1 diabetes in a
consensus analysis of 767 multiplex families. Am J Hum Genet 69:820-830,
2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11507694&query_hl=153
147. Guo D, Li M, Zhang
Y, Yang P, Eckenrode S, Hopkins D, Zheng W, Purohit S, Podolsky RH, Muir A,
Wang J, Dong Z, Brusko T, Atkinson M, Pozzilli P, Zeidler A, Raffel LJ, Jacob
CO, Park Y, Serrano-Rios M, Larrad MT, Zhang Z, Garchon HJ, Bach JF, Rotter JI,
She JX, Wang CY. A functional variant of
SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. Nat
Genet. 2004, 36: 837-41 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15247916&query_hl=155
148. Steck AK, Bugawan TL, Valdes AM, Emery LM, Blair
A, Norris JM, Redondo MJ, Babu SR, Erlich HA, Eisenbarth GS, Rewers MJ.
Association of Non-HLA Genes With Type 1 Diabetes
Autoimmunity. Diabetes. 2005 Aug;54(8):2482-6.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16046318&query_hl=157
149. Barratt BJ, Payne F, Lowe CE, Hermann R, Healy
BC, Harold D, Concannon P, Gharani N, McCarthy MI, Olavesen MG, McCormack R,
Guja C, Ionescu-Tirgoviste C, Undlien DE, Ronningen KS, Gillespie KM,
Tuomilehto-Wolf E, Tuomilehto J, Bennett ST, Clayton DG, Cordell HJ, Todd JA. Remapping the insulin gene/IDDM2 locus in type 1 diabetes.Diabetes.
2004, 53: 1884-9 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15220214&query_hl=158
150. Kavvoura FK, Ioannidis JP. CTLA-4 Gene
Polymorphisms and Susceptibility to Type 1 Diabetes Mellitus: A HuGE Review and
Meta-Analysis.Am J Epidemiol. 2005,162:3-16. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15961581&query_hl=160
151. Ueda H, Howson JM, Esposito L, Heward J, Snook
H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH,
Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S,
Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme
J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A,
Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM,
Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP,
Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd
JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with
susceptibility to autoimmune disease. Nature. 2003,
423: 506-11 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12724780&query_hl=162
152. Bottini N, Musumeci L, Alonso A, Rahmouni S,
Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M,
Eisenbarth GS, Comings D, Mustelin T. A functional variant of lymphoid tyrosine
phosphatase is associated with type I diabetes. Nat Genet. 2004, 36: 337-8 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15004560&query_hl=164
153. Dahlman I, Eaves IA, Kosoy R, Morrison VA,
Heward J, Gough SC, Allahabadia A, Franklyn JA, Tuomilehto J, Tuomilehto-Wolf
E, Cucca F, Guja C, Ionescu-Tirgoviste C, Stevens H, Carr P, Nutland S,
McKinney P, Shield JP, Wang W, Cordell HJ, Walker N, Todd JA, Concannon
P. Parameters for reliable results in genetic association studies in
common disease. Nat Genet 30:149-150, 2002.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11799396&query_hl=167
154. Kaprio J, Tuomilehto J, Koskenvuo M, Romanov K,
Reunanen A, Eriksson J, Stengard J, Kesaniemi YA. Concordance for type 1
(insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a
population-based cohort of twins in
155. Rewers M, Zimmet P. The rising tide of childhood
type 1 diabetes--what is the elusive environmental trigger?Lancet.
2004 Nov 6-12;364(9446):1645-7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15530607&query_hl=170
156. Pak CY, Hyone-Myong E, McArthur RG, Yoon JW . Association of cytomegalovirus
infection with autoimmune type 1 diabetes. Lancet 2:1-3, 1988.
157. Sairenji T, Daibata M, Sorli CH, et al.
Relating homology between the Epstein-Barr virus BOLF1
and HLA-DQw8 beta chain to recent onset type 1 -dependent) diabetes
mellitus. Diabetologia 34:33-39, 1991.
158. Helmke K, Otten A, Willems WR, Brockhaus R,
Mueller-Eckhardt G, Stief T, Bertrams J, Wolf H, Federlin K. Islet cell antibodies and the development of diabetes mellitus in
relation to mumps infection and mumps vaccination. Diabetologia
29:30-33, 1986.
159. Hyoty H, Hiltunen M, Reunanen A, Leinikki P,
Vesikari T, Lounamaa R, Tuomilehto J, Akerblom HK. Decline
of mumps antibodies in type 1 (insulin-dependent) diabetic children and a
plateau in the rising incidence of type 1 diabetes after introduction of the
mumps-measles-rubella vaccine in
160. Ginsberg-Fellner F, Witt ME, Yagihashi S,
Dobersen MJ, Taub F, Fedun B, Mcevoy RC, Roman SH, Davies RG, Cooper LZ, et
al. Congenital rubella syndrome as a model for type 1 (insulin- dependent)
diabetes mellitus: increased prevalence of islet cell surface antibodies.
Diabetologia 27 Suppl:87-89, 1984.
161. Menser MA, Forrest JM,
162. Suenaga KYoon JW. Association
of beta-cell-specific expression of endogenous retrovirus with development of
insulitis and diabetes in NOD mouse. Diabetes 37:1722-1726, 1988.
163. Conrad B, Weidmann E, Trucco G, Rudert WA,
Behboo R, Ricordi C, Rodriquez-Rilo H, Finegold D, Trucco M. Evidence for superantigen involvement in insulin-dependent diabetes
mellitus aetiology. Nature 371:351- 355,
1994.
164. Honeyman MC, Coulson BS, Stone NL, Gellert SA,
Goldwater PN, Steele CE, Couper JJ, Tait BD, Colman PG, Harrison
LC. Association between rotavirus infection and
pancreatic islet autoimmunity in children at risk of developing type 1
diabetes. Diabetes 49:1319-1324, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10923632&query_hl=172
165. Oldstone MB, Nerenberg M, Southern P, Price J,
Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in
a transgenic model: role of anti-self (virus) immune response. Cell
65:319-331, 1991.
166. Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi
CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of "tolerance" and induction of diabetes by virus
infection in viral antigen transgenic mice. Cell 65:305-317, 1991.
167. Bodansky HJ, Dean BM, Bottazzo GF, Grant PJ , McNally J, Hambling MH. Islet-cell
antibodies and insulin autoantibodies in association with common viral
infections. Lancet Dec 13:1351-1353, 1986.
168. Champsaur HF, Bottazzo GF, Bertrams J, Assan R,
Bach C. Virologic, immunologic, and genetic factors in
insulin-dependent diabetes mellitus. J Pediatr 100:15-20, 1982.
169. Uriarte A, Cabrera
E, Ventura R, Vargas J. Islet cell antibodies and ECHO-4 virus infection. Diabetologia 30:590A-1987.
170. Blom L, Nystrom L, Dahlquist G. The Swedish childhood diabetes study. Vaccinations
and infections as risk determinants for diabetes in childhood.
Diabetologia 34:176-181, 1991.
171. Karounos DG, Wolinsky JS, Thomas JW.
Monoclonal antibody to rubella virus capsid protein recognizes a b-cell
antigen. J Immunol 150:3080-3085, 1993.
172. Rewers MAtkinson M. The
possible role of enteroviruses in diabetes mellitus.
353-385, 1996.
173. Graves PM, Norris JM, Pallansch MA, Gerling IC,
Rewers M. The role of enteroviral infections in the development of IDDM:
limitations of current approaches. Diabetes 46: 161-168, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9000690&query_hl=174
174. Jenson AB,
Rosenberg HS, Notkins AL. Pancreatic islet-cell damage in children with fatal
viral infections. Lancet
Aug 16:354-358, 1980.
175. Yoon JW, Austin M, Onodera T, Notkins AL . Virus-induced diabetes mellitus: Isolation of a
virus from the pancreas of a child with diabetic ketoacidosis. N Engl J
Med 300:1173-1179, 1979.
176. Donsky JMassad R. Community
medicine in the training of family physicians. J Fam Pract
8:965-971, 1979.
177. Wagenknecht LE, Roseman JM, Herman WH. Increased incidence of insulin-dependent diabetes mellitus
following an epidemic of coxsackievirus B5. Am J Epidemiol
133:1024-1031, 1991.
178. Kaufman DL, Clare-Salzler M, Tian J, Forsthuber
T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase
in murine insulin-dependent diabetes [see comments]. Nature 366:69-72, 1993.
179. Scherbaum WA, Hampl W, Muir P, Gluck M, Seibler
J, Egle H, Hauner H, Boehm BO, Heinze E, Banatvala JE, Pfeiffer EF . No
association between islet cell antibodies and coxsackie B, mumps, rubella and
cytomegalovirus antibodies in non-diabetic individuals aged 7-19 years.
Diabetologia 34:835-838, 1991.
180. Hyoty H, Hiltunen M, Knip M, Laakkonen M,
Vahasalo P, Karjalainen J, Koskela P, Roivainen M, Leinikki P, Hovi T, . A prospective study of the role of coxsackie B and other
enterovirus infections in the pathogenesis of IDDM. Childhood
Diabetes in
181. Lonnrot M, Korpela K, Knip M, Ilonen J, Simell
O, Korhonen S, Savola K, Muona P, Simell T, Koskela P, Hyoty H.
Enterovirus infection as a risk factor for beta-cell autoimmunity in a
prospectively observed birth cohort: the Finnish Diabetes Prediction and
Prevention Study. Diabetes 49:1314-1318, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10923631&query_hl=179
182. Lonnrot M, Salminen
K, Knip M, Savola K, Kulmala P, Leinikki P, Hyypia T, Akerblom HK, Hyoty
H. Enterovirus RNA in serum is a
risk factor for beta-cell autoimmunity and clinical type 1 diabetes: a
prospective study. Childhood Diabetes in
183. Dahlquist G, Frisk
G, Ivarsson SA, Svanberg L, Forsgren M, Diderholm H. Indications that maternal coxsackie
B virus infection during pregnancy is a risk factor for childhood-onset IDDM. Diabetologia 38:1371-1373, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8582549&query_hl=183
184. Dahlquist GG. Viruses and other perinatal
exposures as initiating events for beta- cell destruction. Ann Med
29:413-417, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9453289&query_hl=185
185. Viskari H,
Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G,
Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Kaplan B, Laron Z,
Koskela P, Vesikari T, Huhtala H, Knip M, Hyoty H. Relationship between the
incidence of type 1 diabetes and maternal enterovirus antibodies: time trends
and geographical variation. Diabetologia. 2005 Jul;48(7):1280-7. Epub 2005 May
19 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15902401&query_hl=187
186. Akerblom HK,
Virtanen SM, Ilonen J, Savilahti E, Vaarala O, Reunanen A, Teramo K, Hamalainen
AM, Paronen J, Riikjarv MA, Ormisson A, Ludvigsson J, Dosch HM, Hakulinen T,
Knip M; National TRIGR Study Groups. Dietary
manipulation of beta cell autoimmunity in infants at increased risk of type 1
diabetes: a pilot study. Diabetologia. 2005 May;48(5):829-37. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15838685&query_hl=189
187. Frisk G, Tuvemo T. Enterovirus infection with
beta-cell tropic strains are frequently in siblings of children diagnosed with
type 1 diabete and in association with elevated levels of GAAAD65 antibodies. J
Med Virol 2004, 73: 450-9 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15170642&query_hl=191
188. Viskari H, Ludvigsson J, Uibo R, Salur L,
Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova
A, Romanov A, Knip M, Hyoty H. Relationship between the incidence of type 1
diabetes and enterovirus infections in different European populations: results
from the EPIVIR project. J Med Virol. 2004 Apr;72(4):610-7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14981763&query_hl=187
189. Devendra D, Eisenbarth GS. Interferon alpha--a potential link in the pathogenesis of
viral-induced type 1 diabetes and autoimmunity. Clin Immunol. 2004
Jun;111(3):225-33. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15183143&query_hl=194
190. Huang X, Yuang J,
Goddard A, Foulis A, James RF, Lernmark A, Pujol-Borrell R, Rabinovitch A,
Somoza N, Stewart TA. Interferon
expression in the pancreases of patients with type I diabetes.Diabetes. 1995
Jun;44(6):658-64. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7540571&query_hl=196
191. Devendra D, Jasinski J, Melanitou E, Nakayama M,
Li M, Hensley B, Paronen J, Moriyama H, Miao D, Eisenbarth GS, Liu E.
Interferon-alpha as a Mediator of Polyinosinic:Polycytidylic
Acid-Induced Type 1 Diabetes. Diabetes. 2005 Sep;54(9):2549-56. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16123342&query_hl=198
192. Graves PM, Rotbart HA,
193.
194. Patterson CC,
195. Stene LC, Barriga K, Norris JM, Hoffman M,
Erlich HA, Eisenbarth GS, McDuffie RS Jr, Rewers M. Perinatal factors and
development of islet autoimmunity in early childhood: the diabetes autoimmunity
study in the young. Am J Epidemiol. 2004 Jul 1;160(1):3-10. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15229111&query_hl=204
196. Rothwell PM, Staines A, Smail P,
197. Oldstone MB. Viruses as
therapeutic agents. I. Treatment of nonobese insulin- dependent diabetes
mice with virus prevents insulin-dependent diabetes mellitus while maintaining
general immune competence. J Exp Med 171:2077-2089, 1990.
198. Wilberz S, Partke
HJ, Dagnaes-Hansen F, Herberg L. Persistent
MHV (mouse hepatitis virus) infection reduces the incidence of diabetes
mellitus in non-obese diabetic mice. Diabetologia 34:2-5, 1991.
199. Like AA, Guberski DL,
200. Kolb HElliott RB. Increasing incidence of
IDDM a consequence of improved hygiene? Diabetologia 37:729-1994.
201. McKinney PA, Okasha M, Parslow RC, Law GR,
Gurney KA, Williams R, Bodansky HJ. Early social mixing and childhood
Type 1 diabetes mellitus: a case- control study in
202. EURODIAB Substudy 2 Study Group Infections and
vaccinations as risk factors for childhood type I (insulin-dependent) diabetes
mellitus: a multicentre case-control investigation..
Diabetologia
43:47-53, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10663215&query_hl=214
203. Bach JF. The effect of infections on
susceptibility to autoimmune and allergic diseases.N Engl J Med. 2002 Sep 19;347(12):911-20 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12239261&query_hl=218
204. Heijbel H, Chen RT,
Dahlquist G. Cumulative incidence
of childhood-onset IDDM is unaffected by pertussis immunization. Diabetes
Care 20:173-175, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9118767&query_hl=220
205. Lindberg B, Ahlfors K, Carlsson A, Ericsson UB,
Landin-Olsson M, Lernmark A, Ludvigsson J, Sundkvist G, Ivarsson SA.
Previous exposure to measles, mumps, and rubella--but not vaccination during
adolescence--correlates to the prevalence of pancreatic and thyroid
autoantibodies. Pediatrics 104:e12-1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10390298&query_hl=222
206. Graves PM, Barriga KJ, Norris JM, Hoffman MR, Yu
L, Eisenbarth GS, Rewers M. Lack of association between
early childhood immunizations and beta-cell autoimmunity. Diabetes
Care 22:1694-1697, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10526737&query_hl=224
207. Hummel M, Fuchtenbusch M, Schenker M, Ziegler
AG. No major association of breast-feeding, vaccinations, and childhood
viral diseases with early islet autoimmunity in the German BABYDIAB
Study. Diabetes Care 23 :969-974, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10895848&query_hl=227
208. Hviid A., Stellfeld M., Wohlfahrt J., Melbye
M.Childhood Vaccination and Type 1 Diabetes. N Engl J Med 2004;
350:1398-1404, Apr 1, 2004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15070789&query_hl=230
209. Atkinson MA, Winter
WE, Skordis N, Beppu H, Riley WM, Maclaren NK. Dietary protein restriction reduces the frequency and
delays the onset of insulin dependent diabetes in BB rats. Autoimmunity
2:11-20, 1988.
210. Martin JM, Trink B, Daneman D, Dosch H-M,
Robinson B. Milk proteins in the etiology of
insulin-dependent diabetes mellitus (IDDM). Ann Med 23:447-452, 1991.
211. Atkinson MA, Bowman MA, Kuo-Jang K,
212. Lampasona V, Ferrari M, Bosi E, Pastore MR,
Bingley PJ, Bonifacio E. Sera from patients with IDDM and healthy
individuals have antibodies to ICA69 on western blots but do not
immunoprecipitate liquid phase antigen. J Autoimmun 7:665-674,
1994.
213. Martin S, Lampasona
V, Dosch M, Pietropaolo M. Islet cell autoantigen 69 antibodies in
IDDM. Diabetologia 39:747-1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8781774&query_hl=232
214. Krokowski M,
Caillat-Zucman S, Timsit J, Larger E, Pehuet-Figoni M, Bach JF, Boitard
C. Anti-bovine serum albumin
antibodies: Genetic heterogeneity and clinical relevance in adult-onset
IDDM. Diabetes Care 18:170-173, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7729293&query_hl=235
215. Norris JM, Beaty B, Klingensmith G, Yu L,
Hoffman M, Chase HP, Erlich HA, Hamman RF, Eisenbarth GS, Rewers M. Lack
of association between early exposure to cow's milk protein and beta-cell
autoimmunity: Diabetes Autoimmunity Study in the Young (DAISY). J A M A 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8773632&query_hl=238
216. Virtanen SM, Hypponen E, Laara E, Vahasalo P,
Kulmala P, Savola K, Rasanen L, Aro A, Knip M, Akerblom HK. Cow's milk
consumption, disease-associated autoantibodies and type 1 diabetes mellitus: a
follow-up study in siblings of diabetic children. Childhood
Diabetes in Finland Study Group. Diabet Med 15:730-738, 1998. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9737801&query_hl=240
217. Couper JJ, Steele C, Beresford S, Powell T ,
McCaul K, Pollard A, Gellert S, Tait B, Harrison LC, Colman PG. Lack of association between duration of breast-feeding or
introduction of cow's milk and development of islet autoimmunity. Diabetes
48:2145-2149, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10535447&query_hl=242
218. Borch-Johnsen K,
Joner G, Mandrup-Poulsen T, Christy M, Zachau-Christiansen B, Kastrup K, Nerup
J. Relation between breast-feeding
and incidence rates of insulin-dependent diabetes mellitus. Lancet
2:1083-1086, 1984.
219. Mayer EJ, Hamman
RF, Gay EC, Lezotle DC, Savitz DA, Klingensmith GJ. Reduced risk of IDDM among
breast-fed children. The
220. Kostraba JN, Dorman JS,
LaPorte RE, Scott FW,
221. Nigro G, Campea L, De Novellis A, Orsini
M. Breast-feeding and insulin-dependent diabetes
mellitus. Lancet 1: 467 (Letter)-1985.
222. Blom L, Dahlquist G, Nystrom L, Sandstrom A,
Wall S. The Swedish childhood diabetes study - social
and perinatal determinants for diabetes in childhood. Diabetologia
32:7-13, 1989.
223. Kyvik KO, Green A, Svendsen A, Mortensen
K. Breast feeding and the development of type I
diabetes mellitus. Diabetic Med 9:233-235, 1992.
224. Norris JM, Barriga K, Klingensmith G, Hoffman M,
Eisenbarth GS, Erlich HA, Rewers M. Timing of initial cereal exposure in
infancy and risk of islet autoimmunity. JAMA. 2003 Oct
1;290(13):1713-20. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14519705&query_hl=244
225. Virtanen SM, Rasanen L, Aro A, Ylonen K,
Lounamaa R, Tuomilehto J, Akerblom HK, the 'Childhood Diabetes in Finland'
Study Group. Feeding in infancy and the risk of type 1
diabetes mellitus in Finnish children. Diabetic Med 9:815-819, 1992.
226. Gerstein HC. Cow's milk exposure and type
I diabetes mellitus - a critical overview of the clinical literature.
Diabetes Care 17:13-19, 1994.
227. Norris JM, Scott FW. A meta-analysis of
infant diet and insulin-dependent diabetes mellitus: do biases play a
role? Epidemiology 7:87-92, 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8664407&query_hl=246
228. Savilahti E, Akerblom HK, Tainio VM, Koskimies
S. Children with newly diagnosed insulin dependent diabetes mellitus have
incresed levels of cow's milk antibodies. Diabetes Res 7:137-140, 1988.
229. Karjalainen J, Martin JM, Knip M, Ilonen J,
Robinson BH, Savilahti E, Akerblom HK, Dosch HM. A
bovine albumin peptide as a possible trigger of insulin- dependent diabetes
mellitus [see comments]. N Engl J Med 327:302-307, 1992.
230. Virtanen SM, Laara E, Hypponen E, Reijonen H,
Rasanen L, Aro A, Knip M, Ilonen J, Akerblom HK. Cow's milk consumption,
HLA-DQB1 genotype, and type 1 diabetes: a nested case-control study of siblings
of children with diabetes. Childhood diabetes in
231. Norris JM, Barriga K, Klingensmith G, Hoffman M,
Eisenbarth GS, Erlich HA, Rewers M. Timing of initial cereal exposure in
infancy and risk of islet autoimmunity. JAMA. 2003 Oct
1;290(13):1713-20. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14519705&query_hl=250
232. Ziegler AG, Schmid S, Huber D, Hummel M,
Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA.
2003 Oct 1;290(13):1721-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14519706&query_hl=251
233. Fronczak CM, Baron AE, Chase HP, Ross C, Brady
HL, Hoffman M, Eisenbarth GS, Rewers M, Norris JM. In utero
dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003 Dec;26(12):3237-42.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14633808&query_hl=253
234. Hyppönen E, Läärä E, Reunanen A, Jarvelin M-R,
Virtanen SM. Intake of vitamin D and risk of type 1diabetes: a birth cohort
study. Lancet 2001, 358:1500-1503 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11705562&query_hl=255
235. Stene LC, Joner G; Norwegian Childhood Diabetes
Study Group. Use of cod liver oil during the first year of life is associated
with lower risk of childhood-onset type 1 diabetes: a large, population-based,
case-control study. Am J Clin Nutr. 2003 Dec;78(6):1128-34 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14668274&query_hl=257
236. Muntoni S, Karvonen M, Muntoni S, Tuomilehto J.
Seasonality of birth in patients with type 1 diabetes. Lancet. 2002 Apr
6;359(9313):1246 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11955561&query_hl=259
237. Laron Z, Lewy H, Wilderman I, Casu A, Willis J,
Redondo MJ, Libman I, White N, Craig M. Seasonality of month of birth of
children and adolescents with type 1 diabetes mellitus in homogenous and
heterogeneous populations. Isr Med Assoc J. 2005 Jun;7(6):381-4.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15984382&query_hl=261
238. Weets I, Kaufman L, Van der Auwera B, Crenier L,
Rooman RP, De Block C, Casteels K, Weber E, Coeckelberghs M, Laron Z, Pipeleers
DG, Gorus FK; Belgian Diabetes Registry. Seasonality in clinical onset of type
1 diabetes in belgian patients above the age of 10 is
restricted to HLA-DQ2/DQ8-negative males, which explains the male to female
excess in incidence. Diabetologia. 2004 Apr;47(4):614-21 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15298337&query_hl=263
239. Kantwerk-Funke G, Burkart V, Kolb H. Low dose
streptozotocin causes stimulation of the immune system and of anti-islet
cytotoxicity in mice. Clin Exp Immunol. 1991 Nov;86(2):266-70.
240. Elias D, Prigozin H, Polak N, Rapoport M, Lohse
AW, Cohen IR. Autoimmune diabetes induced by the beta-cell toxin STZ. Immunity to the 60-kDa heat shock protein and to insulin.
Diabetes 43:992-998, 1994.
241. Rayfield EJIshimura K. Environmetal
factors and insulin dependent diabetes mellitus. Diabetes Metab
Rev 3:925-957, 1987.
242. Helgason TJonasson MR. Evidence
for a food additive as a cause of ketosis-prone diabetes. Lancet 2:716-720,
1981.
243. Dahlquist GG, Blom
LG, Persson L-Å, Sandström AIM, Wall SGI. Dietary factors and the risk of developing insulin
dependent diabetes in childhood.
Br Med J
300:1302-1306, 1990.
244. Kostraba JN, Gay
EC, Rewers M, Hamman RF. Nitrate
levels in community drinking waters and risk of IDDM. Diabetes Care
15:1505-1508, 1992.
245. Toniolo A, Onodera T, Yoon JW, Notkins AL.
Induction of diabetes by cumulative environmental insults
from viruses and chemicals. Nature 288:383-385,
1980.
246. Wilkin TJ. The accelerator hypothesis: weight
gain as the missing link between Type I and Type II diabetes. Diabetologia. 2001
Jul;44(7):914-22. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11508279&query_hl=265
247. Bruining GJ: Association between infant growth
before onset of juvenile type 1 diabetes and autoantibodies to IA-2. Lancet
356: 655–656, 2000 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10968443&query_hl=269
248. Betts P, Mulligan J, Ward P, Smith B, Wilkin T.
Increasing body weight predicts the earlier onset of insulin-dependant diabetes
in childhood: testing the 'accelerator hypothesis' (2). Diabet Med. 2005 Feb;22(2):144-51.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15660730&query_hl=271
249. Stene LC, Magnus P,
Lie RT, Sovik O, Joner G; Norwegian childhood Diabetes Study Group. Birth weight and childhood onset type 1 diabetes:
population based cohort study. BMJ. 2001 Apr 14;322 (7291):889-92. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11302899&query_hl=273
250. Hypponen E, Virtanen SM, Kenward MG, Knip M,
Akerblom HK, The Childhood Diabetes in Finland Study
Group: Obesity, increased linear growth, and risk of type 1 diabetes in
children. Diabetes Care 23: 1755–1760, 2000 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11128347&query_hl=275
251. Larsson HE, Lynch K, Lernmark B, Nilsson A,
Hansson G, Almgren P, Lernmark A, Ivarsson SA; DiPiS Study Group.
Diabetes-associated HLA genotypes affect birthweight in the general population.
Diabetologia.
2005 Jul 1 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15991024&query_hl=277
252. Patterson CC, Dahlquist G, Soltesz G, Green A;
EURODIAB ACE Study Group.
253. Khoury MJ, Flanders WD,
254. Fohlman J, Bohme J, Rask L, Frisk G, Diderholm
H, Friman G, Tuvemo T. Matching of host genotype
and serotypes of Coxsackie B virus in the development of juvenile diabetes.
Scand J Immunol 26:105-110, 1987.
255. D'Alessio DJ. A case-control study of
group B Coxsackievirus immunoglobulin M antibody prevalence and HLA-DR antigens
in newly diagnosed cases of insulin-dependent diabetes mellitus. Am J Epidemiol 135
:1331-1338, 1992.
256. Schernthaner G,
Banatvala JE, Scherbaum W, Bryant J, Borkenstein M, Schober E, Mayr WR. Coxsackie-B-virus-specific IgM responses,
complement-fixing islet-cell antibodies, HLA DR antigens,
and C-peptide secretion in insulin-dependent diabetes mellitus. Lancet
2:630-632, 1985.
257. Kostraba JN, Cruickshanks KJ, Lawler-Heavner J,
Jobim LF, Rewers MJ, Gay EC , Chase HP, Klingensmith G, Hamman RF. Early exposure to cow's milk and solid foods in infancy, genetic
predisposition and risk of IDDM. Diabetes 42:288-295, 1993.
258. Norris JM, Beaty B, Klingensmith G, Yu L,
Hoffman M, Chase HP, Erlich HA, Hamman RF, Eisenbarth GS, Rewers M. Lack
of association between early exposure to cow's milk protein and b-cell
autoimmunity: Diabetes Autoimmunity Study in the Young (DAISY). JAMA
276:609-614, 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8773632&query_hl=284
259. Knip M, Sakkinen A, Huttunen NP, Kaar ML,
Lankela S, Mustonen A, Akerblom HK. Postinitial
remission in diabetic children--an analysis of 178 cases. Acta
Paediatr Scand 71:901-908, 1982.
260. Agner T, Damm P,
Binder C. Remission in IDDM:
prospective study of basal C-peptide and insulin dose in 268 consecutive
patients. Diabetes Care 10:164-169, 1987.
261. Sochett EB, Daneman D, Clarson
C,
262. Ortqvist E, Falorni
A, Scheynius A, Persson B, Lernmark A. Age governs gender-dependent islet cell autoreactivity and predicts the
clinical course in childhood IDDM. Acta Paediatr 86 :1166-1171, 1997.
263. Schiffrin A, Suissa
S, Poussier P, Guttmann R, Weitzner G. Prospective study of predictors of beta-cell survival in type I
diabetes. Diabetes 37:920-925, 1988.
264. Wallensteen M, Dahlquist G, Persson B,
Landin-Olsson M, Lernmark A, Sundkvist G, Thalme B. Factors influencing
the magnitude, duration, and rate of fall of B-cell function in Type I
(insulin-dependent) diabetic children followed for two years from their
clinical diagnosis. Diabetologia 31:664-669, 1988.
265. Sabbah E, Savola K, Kulmala P, Veijola R, Vahasalo
P, Karjalainen J, Akerblom HK, Knip M. Diabetes-associated autoantibodies
in relation to clinical characteristics and natural course in children with
newly diagnosed type 1 diabetes. The Childhood Diabetes In
Finland Study Group. J Clin Endocrinol Metab 84:1534-1539, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10323375&query_hl=287
266. Petersen JS, Dyrberg T, Karlsen AE, Molvig J,
Michelsen B, Nerup J, Mandrup-Poulsen T. Glutamic acid
decarboxylase (GAD65) autoantibodies in prediction of B-cell function and
remission in recent-onset IDDM after cyclosporin treatment. Diabetes
43:1291-1296, 1994.
267. Fukuda M, Tanaka A, Tahara Y, Ikegami H,
Yamamoto Y, Kumahara Y, Shima K. Correlation between
minimal secretory capacity of pancreatic beta-cells and stability of diabetic
control. Diabetes 37:81-88, 1988.
268. Kolb H, Dannehl K, Gruenklee D, Zielasek J,
Bertrams J, Hubinger A, Gries FA. Prospective analysis of islet cell
antibodies in children with type I (insulin-dependent) diabetes.
Diabetologia 31:189-194, 1988.
269. Knip M, Llonen J, Mustonen A, Akerblom HK.
Evidence of an accelerated B-cell destruction in HLA-Dw3/Dw4
heterozygous children with type I (insulin-dependent) diabetes.
Diabetologia 29:347-351, 1986.
270. Pipeleers DLing Z. Pancreatic
beta cells in insulin-dependent diabetes. Diabetes Metab Rev 8:209-227, 1992.
271. Madsbad S, Faber OK, Binder C, McNair P,
Christiansen C, Transbol I. Prevalence of residual beta-cell function in insulin-dependent diabetics in relation to age
at onset and duration of diabetes. Diabetes 27(Suppl. 1):262-264, 1978.
272. Rewers A, Chase HP, Mackenzie T, Walravens P,
Roback M, Rewers M, Hamman RF, Klingensmith G. Predictors of acute
complications in children with type 1 diabetes. JAMA.
2002 May 15;287(19):2511-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12020331&query_hl=289
273. Maniatis AK, Goehrig SH, Gao
D, Rewers A, Walravens P, Klingensmith GJ. Increased
incidence and severity of diabetic ketoacidosis among uninsured children with
newly diagnosed type 1 diabetes mellitus. Pediatr Diabetes. 2005 Jun;6(2):79-83. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15963034&query_hl=291
274. Dorman JS, LaPorte RE, Kuller LH, Cruickshanks
KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL. The
275. Cusick M, Meleth AD, Agron E, Fisher MR, Reed
GF, Knatterud GL, Barton FB, Davis MD, Ferris FL 3rd, Chew EY; Early Treatment
Diabetc Retinopathy Study Research Group. Associations of mortality and
diabetes complications in patients with type 1 and type 2 diabetes: early
treatment diabetic retinopathy study report no. 27. Diabetes
Care. 2005 Mar;28(3):617-25. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15735198&query_hl=293
276. Nishimura R, LaPorte RE, Dorman JS, Tajima N,
Becker D, Orchard TJ. Mortality trends in type 1 diabetes. The
277. Skrivarhaug T, Sandvik L, Joner G. Long-term mortality
in nationwide cohort of childhood-onset type 1 diabetes in
278. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen
H. Mortality and causes of death in the WHO Multinational Study of Vascular
Disease in Diabetes.Diabetologia. 2001 Sep;44 Suppl
2:S14-21. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11587045&query_hl=304
279. Laing SP, Swerdlow AJ, Carpenter LM, Slater SD,
Burden AC, Botha JL, Morris AD, Waugh NR, Gatling W, Gale EA, Patterson CC,
Qiao Z, Keen H. Mortality from cerebrovascular disease in a cohort of 23,000
patients with insulin-treated diabetes. Stroke. 2003
Feb;34(2):418-21. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12574553&query_hl=306
280. Swerdlow AJ, Laing SP, Dos Santos Silva I,
Slater SD, Burden AC, Botha JL, Waugh NR, Morris AD, Gatling W, Bingley PJ,
Patterson CC, Qiao Z, Keen H. Mortality of South Asian patients with
insulin-treated diabetes mellitus in the United Kingdom: a cohort study. Diabet Med. 2004
Aug;21(8):845-51. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15270787&query_hl=308
281. Laing SP, Swerdlow AJ, Slater SD, Burden AC,
Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC. Mortality from heart
disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003 Jun;46(6):760-5.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12774166&query_hl=310
282. Major cross-country differences in risk of dying
for people with IDDM. Diabetes Epidemiology Research International Mortality
Study Group. Diabetes Care 14:49-54, 1991.
283. Matsushima M, LaPorte RE, Maruyama M,
284. International evaluation of cause-specific
mortality and IDDM. Diabetes Epidemiology Research International Mortality
Study Group. Diabetes Care 14:55-60, 1991.
285. Borch-Johnsen K, Nissen H, Henriksen E, Kreiner
S, Salling N, Deckert T, Nerup J. The natural history of
insulin-dependent diabetes mellitus in
286. Fuller JH, Stevens LK, Wang SL. Risk
factors for cardiovascular mortality and morbidity: the WHO Mutinational Study
of Vascular Disease in Diabetes. Diabetologia. 2001
Sep;44 Suppl 2:S54-64. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11587051&query_hl=314
287. Orchard TJ, Olson JC, Erbey JR, Williams K,
Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin
resistance-related factors, but not glycemia, predict coronary artery disease
in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of
Diabetes Complications Study.Diabetes Care. 2003 May;26(5):1374-9
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12716791&query_hl=316
288. Chowdhury TA, Dyer PH, Mijovic CH, Dunger DB,
Barnett AH,
289. Doria A, Warram JH,
290. Doria A, Onuma T, Gearin G,
291. Fogarty DG, Harron JC, Hughes AE, Nevin NC,
Doherty CC, Maxwell AP. A molecular variant of angiotensinogen is
associated with diabetic nephropathy in IDDM. Diabetes 45:1204-1208,
1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8772723&query_hl=322
292. Nagi DK, Mansfield MW, Stickland MH, Grant
PJ. Angiotensin converting enzyme (ACE)
insertion/deletion (I/D) polymorphism, and diabetic retinopathy in subjects
with IDDM and NIDDM. Diabet Med 12:997-1001, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8582133&query_hl=324
293. Parving HH, Jacobsen P,
294.
295. Tarnow L, Cambien F, Rossing P, Nielsen FS ,
Hansen BV, Lecerf L, Poirier O, Danilov S, Parving HH. Lack
of relationship between an insertion/deletion polymorphism in the angiotensin
I-converting enzyme gene and diabetic nephropathy and proliferative retinopathy
in IDDM patients. Diabetes 44:489-494, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7729604&query_hl=330
296. Ruiz J, Blanche H,
James RW, Garin MC, Vaisse C, Charpentier G, Cohen N, Morabia A, Passa P,
Froguel P. Gln-Arg192
polymorphism of paraoxonase and coronary heart disease in type 2 diabetes [see
comments]. Lancet 346:869-872, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7564671&query_hl=332
297. Garin MC, James RW, Dussoix P, Blanche H, Passa
P, Froguel P, Ruiz, J. Paraoxonase polymorphism Met-Leu54 is associated
with modified serum concentrations of the enzyme. A possible
link between the paraoxonase gene and increased risk of cardiovascular disease
in diabetes. J Clin Invest 99:62-66, 1997.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9011577&query_hl=335
298. Rewers M, Kamboh MI, Hoag S, Shetterly SM,
Ferrell RE, Hamman RF. Apolipoprotein A-IV polymorphism associated with
myocardial infarction in obese NIDDM patients. The
299. Retinopathy and Nephropathy in Patients with
Type 1 Diabetes Four Years after a Trial of Intensive Therapy. N Engl J
Med 342:381-389, 0 AD.
300. Matsushima M, LaPorte RE, Maruyama M,
301. Songer TJ, LaPorte R, Lave JR, Dorman JS, Becker
DJ. Health insurance and the financial impact of IDDM
in families with a child with IDDM. Diabetes Care 20:577-584, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9096983&query_hl=338
302. Javitt JC, Chiang
Y-P. Economic
impact of diabetes.
2:601-611, 1995.
303. Portuese E, Orchard TJ. Mortality in insulin-dependent diabetes. 2:221-232, 1995.
304. The Diabetes Control and Complications Trial
Research Group. The effect of intensive treatment of
diabetes on the development and progression of long-term complications in
insulin-dependent diabetes mellitus. New Engl J Med 329:977-986, 1993.
305. The Diabetes Control and Complications Trial
Research Group. The relationship of glycemic exposure
(HbA1c) to the risk of development and progression of retinopathy in the
diabetes control and complications trial. Diabetes 44:968-983 ,
1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7622004&query_hl=352
306. Retinopathy and nephropathy in patients with
type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of
Diabetes Interventions and Complications Research Group. N Engl J
Med 342:381-389, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10793172&query_hl=354
307. DCCT. Epidemiology of
severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med 90:450-459, 1991.
308. The Diabetes Control and Complications Trial
Research Group. Adverse events and their association
with treatment regimens in the diabetes control and complications trial.
Diabetes Care 18:1415-1427, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8722064&query_hl=356
309. Klein R, Klein BEK, Moss SE, et al. The
310. Mizutani M, Kern TS, Lorenzi M.
Accelerated death of retinal microvascular cells in human and experimental
diabetic retinopathy. J Clin Invest 97:2883-2890,
1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8675702&query_hl=1
311. Konno S, Feke GT, Yoshida A, Fujio N, Goger DG,
Buzney SM. Retinal blood flow changes in type I diabetes. A long-term follow-up study. Invest Ophthalmol Vis Sci
37:1140-1148, 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8631628&query_hl=3
312. Bursell SE, Clermont AC, Kinsley BT, Simonson
DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with
insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis
Sci 37:886-897, 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8603873&query_hl=5
313. Mori F, Konno S,
Hikichi T, Yamaguchi Y, Ishiko S, Yoshida A. Pulsatile ocular blood flow study: decreases in
exudative age related macular degeneration. Br J Ophthalmol 85:531-533, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11316708&query_hl=7
314. Feke GT, Buzney SM, Ogasawara H, Fujio N, Goger
DG, Spack NP, Gabbay KH. Retinal circulatory
abnormalities in type 1 diabetes. Invest Ophthalmol Vis Sci
35:2968-2975, 1994.
315. Clermont AC, Aiello LP, Mori F, Aiello LM,
Bursell SE. Vascular endothelial growth factor and severity of
nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a
potential role for vascular endothelial growth factor in the progression of
nonproliferative diabetic retinopathy. Am J Ophthalmol
124:433-446, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9323935&query_hl=9
316. Andersen AR,
Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy
in Type 1 (insulin-dependent) diabetes: an epidemiological study.
Diabetologia 25:496-501, 1983.
317. Krolewski AS,
Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type 1
diabetes. Am J Med
78:785-794, 1985.
318. Klein R, Klein BE,
Moss SE. The
incidence of gross proteinuria in people with insulin- dependent diabetes
mellitus [see comments].
Arch Intern Med 151 :1344-1348, 1991.
319. Kofoed-Enevoldsen A, Borch-Johnsen K, Kreiner S,
Nerup J, Deckert T. Declining incidence of persistent
proteinuria in type I (insulin- dependent) diabetic patients in
320. Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining
incidence of nephropathy in insulin-dependent diabetes mellitus.
New Engl J Med 330:15-18, 1994.
321. Tuomilehto J, Borch-Johnsen K, Molarius A,
Jormanainen V, Lounamaa R, Gronhagen-Riska C, Reunanen A, Sarti C. The unchanging incidence of hospitalization for diabetic
nephropathy in a population-based cohort of IDDM patients in
322. Chavers BM, Bilous RW, Ellis EN, Steffes MW , Mauer SM. Glomerular lesions and urinary albumin
excretion in type I diabetes without overt proteinuria. N Engl J Med
320:966-970, 1989.
323. Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various
levels of albuminuria. Diabetes 43:1358-1364,
1994.
324. Adler SG, Kang SW, Feld S, Cha DR, Barba L,
Striker L, Striker G, Riser BL, LaPage J, Nast CC. Glomerular mRNAs in
human type 1 diabetes: biochemical evidence for microalbuminuria as a
manifestation of diabetic nephropathy. Kidney Int 60:2330-2336, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11737607&query_hl=13
325. Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A,
Mahmud U, Keen H. Microalbuminuria as a predictor of
clinical nephropathy in insulin- dependent diabetes mellitus.
Lancet 1:1430-1432, 1982.
326. Parving HH, Oxenboll B,
Svendsen PA, Sandahl-Christiansen J,
327. Viberti GC, Mogensen CE, Groop LC, Pauls JF , European Microalbuminuria Captopril Study Group. Effect of Captopril on progression to clinical proteinuria in
patients with insulin-dependent diabetes mellitus and microalbuminuria.
J A M A 271:275-279, 1994.
328. Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition
with captopril on diabetic nephropathy in normotensive IDDM patients with
microalbuminuria. North American Microalbuminuria Study Group. Am
J Med 99:497-504, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7485207&query_hl=15
329. Randomised placebo-contr
330. Should all patients with type 1 diabetes
mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors?
A meta-analysis of individual patient data. Ann
Intern Med 134:370-379, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11242497&query_hl=20
331. Caramori ML,
Fioretto P, Mauer M. The need for
early predictors of diabetic nephropathy risk: is albumin excretion rate
sufficient? Diabetes 49:1399-1408, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10969821&query_hl=22
332. Mogensen CEChristensen CK. Predicting diabetic nephropathy in insulin-dependent patients.
N Engl J Med 311:89-93, 1984.
333. Caramori ML, Gross JL, Pecis M, de Azevedo
MJ. Glomerular filtration rate, urinary albumin excretion rate, and blood
pressure changes in normoalbuminuric normotensive type 1 diabetic patients: an 8-year follow-up study. Diabetes Care
22:1512-1516, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10480518&query_hl=24
334. Garg SK, Chase HP, Icaza G, Rothman RL, Osberg
I, Carmain JA. 24-hour ambulatory blood pressure and renal disease in
young subjects with type I diabetes. J Diabetes Complications 11:263-267, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9334907&query_hl=26
335. Lafferty AR,
336. Sundkvist GLilja B. Autonomic neuropathy
predicts deterioration in glomerular filtration rate in patients with
IDDM. Diabetes Care 16:773-779, 1993.
337. Lemley KV, Blouch K, Abdullah I, Boothroyd DB,
Bennett PH, Myers BD, Nelson RG. Glomerular
permselectivity at the onset of nephropathy in type 2 diabetes mellitus.
J Am Soc Nephrol 11:2095-2105, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11053486&query_hl=30
338. Lemley KV, Abdullah I, Myers BD, Meyer TW,
Blouch K, Smith WE, Bennett PH, Nelson RG.
Evolution of incipient nephropathy in type 2 diabetes
mellitus. Kidney Int 58:1228-1237, 2000.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10972685&query_hl=32
339. Mifsud SA, Allen TJ, Bertram JF, Hulthen UL,
Kelly DJ, Cooper ME, Wilkinson-Berka JL, Gilbert RE. Podocyte foot
process broadening in experimental diabetic nephropathy: amelioration with
renin-angiotensin blockade. Diabetologia 44:878-882,
2001 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11508273&query_hl=34
340. Steffes MW, Schmidt D, McCrery R, Basgen JM . Glomerular cell number in
normal subjects and in type 1 diabetic patients. Kidney Int
59:2104-2113, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11380812&query_hl=36
341. Pagtalunan ME, Miller PL, Jumping-Eagle S,
Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II
diabetes. J Clin Invest 99:342-348, 1997.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9006003&query_hl=38
342. Tesfaye S, Chaturvedi N, Eaton SE, Ward JD,
Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH; EURODIAB Prospective
Complications Study Group. Vascular risk factors and diabetic
neuropathy. N Engl J Med. 2005 Jan 27;352(4):341-50.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15673800&query_hl=40
343. Retinopathy and nephropathy in patients with
type 1 diabetes four years after a trial of intensive therapy. The Diabetes
Control and Complications Trial/Epidemiology of Diabetes Interventions and
Complications Research Group.N Engl J Med. 2000 Feb 10;342(6):381-9.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10666428&query_hl=42
344. Klein R, Klein BE, Moss SE, Cruickshanks
KJ. Relationship of hyperglycemia to the long-term
incidence and progression of diabetic retinopathy. Arch Intern Med
154:2169- 2178, 1994.
345. Krolewski AS, Laffel LMB, Krolewski M, Quinn M,
Warram JH. Glycosylated hemoglobin and the risk of
microalbuminuria in patients with insulin-dependent diabetes mellitus.
N Engl J Med 332:1251-1255, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7708068&query_hl=47
346. Orchard TJ, Forrest KY, Ellis D, Becker
DJ. Cumulative glycemic exposure and microvascular
complications in insulin- dependent diabetes mellitus. The glycemic
threshold revisited . Arch Intern Med
157:1851-1856, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9290544&query_hl=49
347. Chase HP,
348. Klein R, Klein BE, Moss SE, Cruickshanks
KJ. Ten-year incidence of gross proteinuria in people
with diabetes. Diabetes 44:916-923, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7621997&query_hl=51
349. Chase HP, Jackson WE, Hoops SL, Cockerham RS,
Archer PG, O'Brien D. Glucose control and the renal and retinal complications
of insulin-dependent diabetes. JAMA. 1989 Feb 24;261(8):1155-60.
350. Porta M, Sjoelie AK, Chaturvedi N, Stevens L,
Rottiers R, Veglio M, Fuller JH. Risk factors for
progression to proliferative diabetic retinopathy in the EURODIAB Prospective
Complications Study. Diabetologia 44:2203-2209, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11793022&query_hl=53
351. Chase HP,
352. Scott LJ, Warram JH, Hanna LS,
Laffel LM, Ryan L,
353. Moss SE, Klein R,
Klein BE. Cigarette
smoking and ten-year progression of diabetic retinopathy. Ophthalmology 103:1438-1442,
1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8841303&query_hl=58
354. Chaturvedi N, Sjoelie AK, Porta M, Aldington SJ,
Fuller JH, Songini M, Kohner EM. Markers of insulin resistance are strong
risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care
24:284-289, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11213880&query_hl=60
355. Chaturvedi N,
Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: rates, risk
factors and glycemic threshold. Kidney Int 60:219-227,
2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11422754&query_hl=62
356.
357. Lam KS, Cheng IK,
Janus ED, Pang RW. Cholesterol-lowering
therapy may retard the progression of diabetic nephropathy. Diabetologia
38:604-609, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7489845&query_hl=66
358. De Cosmo S,
Argiolas A, Miscio G, Thomas S, Piras GP, Trevisan R, Perin PC, Bacci S, Zucaro
L, Margaglione M, Frittitta L, Pizzuti A, Tassi V, Viberti GC, Trischitta
V. A PC-1 amino acid variant
(K121Q) is associated with faster progression of renal disease in patients with
type 1 diabetes and albuminuria. Diabetes 49:521-524, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10868979&query_hl=68
359. Lustman PJ, Freedland KE,
Griffith LS, Clouse RE.
Fluoxetine for depression in diabetes: a randomized double-blind placebo-contr
360. Izuora KE, Chase HP, Jackson WE, Coll JR, Osberg
IM, Gottlieb PA, Rewers MJ, Garg SK. Inflammatory markers and diabetic
retinopathy in type 1 diabetes. Diabetes Care. 2005
Mar;28(3):714-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15735215&query_hl=72
361. Schram MT, Chaturvedi N, Schalkwijk CG, Fuller
JH, Stehouwer CD; EURODIAB Prospective Complications Study Group.Markers of
inflammation are cross-sectionally associated with microvascular complications
and cardiovascular disease in type 1 diabetes--the EURODIAB Prospective
Complications Study.Diabetologia. 2005 Feb;48(2):370-8.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15692810&query_hl=74
362. Jensen T. Increased
plasma concentration of von Willebrand factor in insulin dependent diabetics
with incipient nephropathy. BMJ 298:27-28,
1989.
363. Maser RE, Ellis D, Erbey JR, Orchard TJ.
Do tissue plasminogen activator-plasminogen activator inhibitor-1 complexes relate
to the complications of insulin-dependent diabetes mellitus?
364. Klein R, Klein BE,
Moss SE. The
365. Lustman PJ, Skor DA, Carney
RM,
366.
367. Lustman PJClouse RE. Relationship of
psychiatric illness to impotence in men with diabetes .
Diabetes Care 13: 893-895, 1990.
368. Goldston DB, Kelley AE, Reboussin DM, Daniel SS,
Smith JA, Schwartz RP, Lorentz W, Hill C. Suicidal
ideation and behavior and noncompliance with the medical regimen among diabetic
adolescents. J Am Acad Child Adolesc Psychiatry 36:1528- 1536,
1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10361783&query_hl=81
369. Barker JM, Goehrig SH, Barriga K, Hoffman M,
Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M; DAISY study.
Clinical characteristics of children diagnosed with type 1
diabetes through intensive screening and follow-up. Diabetes
Care. 2004 Jun;27(6):1399-404. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15161795&query_hl=83
370. The Diabetes Control and Complications Trial
Research Group. Effect of intensive therapy on residual
beta-cell function in patients with type 1 diabetes in the diabetes control and
complications trial. A randomized, contr
371. Borch-Johnsen K,
Nissen H, Henriksen E, Kreiner S, Salling N, Deckert T, Nerup J. The natural history of insulin-dependent diabetes
mellitus in
372. Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy.
N Engl J Med 320:1161- 1165, 1989.
373. Borch-Johnsen K,
Norgaard K, Hommel E, Mathiesen ER, Jensen JS, Deckert T, Parving HH. Is diabetic nephropathy an inherited
complication? Kidney Int 41:719-722, 1992.
374. The Diabetes Control and Complications Trial
Research Group. Clustering of long-term complications
in families with diabetes in the diabetes control and complications trial.
The Diabetes Control and Complications Trial Research Group.
Diabetes 46:1829-1839, 1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9356033&query_hl=88
375. Pettitt DJ, Saad MF, Bennett PH, Nelson RG , Knowler WC. Familial predisposition to renal
disease in two generations of Pima Indians with type 2 (non-insulin-dependent)
diabetes mellitus. Diabetologia 33:438-443, 1990.
376. Freedman BI, Tuttle AB, Spray BJ. Familial predisposition to nephropathy in African-Americans with
non- insulin-dependent diabetes mellitus. Am J Kidney Dis
25:710-713, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7747724&query_hl=90
377. Bergman S, Key BO, Kirk KA, Warnock DG, Rostant
SG. Kidney disease in the first-degree relatives of
African-Americans with hypertensive end-stage renal disease. Am J
Kidney Dis 27:341-346, 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8604702&query_hl=92
378. Spray BJ, Atassi
NG, Tuttle AB, Freedman BI. Familial risk, age at onset, and cause of end-stage
renal disease in white Americans.
J Am Soc Nephrol 5:1806-1810, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7787148&query_hl=94
379. O'Dea DF, Murphy SW, Hefferton D, Parfrey
PS. Higher risk for renal failure in first-degree relatives of white
patients with end-stage renal disease: a population-based study
. Am J Kidney Dis 32:794-801, 1998.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9820449&query_hl=96
380. Canani LH, Gerchman
F, Gross JL. Familial
clustering of diabetic nephropathy in Brazilian type 2 diabetic patients. Diabetes 48:909-913, 1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10102711&query_hl=98
381. Fioretto P, Steffes
MW, Barbosa J, Rich SS, Miller ME, Mauer M. Is diabetic nephropathy
inherited? Studies of
glomerular structure in type 1 diabetic sibling pairs. Diabetes 48:865-869,
1999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10102705&query_hl=100
382. Brancati FL, Whittle JC,
383. Fogarty DG, Rich SS, Hanna L, Warram JH,
384. Chowdhury TA, Dyer PH, Mijovic CH, Dunger DB,
Barnett AH,
385. Doria A, Warram JH,
386. Doria A, Onuma T, Gearin G,
387. Fogarty DG, Harron JC, Hughes AE, Nevin NC,
Doherty CC, Maxwell AP. A molecular variant of angiotensinogen is
associated with diabetic nephropathy in IDDM. Diabetes 45:1204-1208,
1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8772723&query_hl=108
388. Nagi DK, Mansfield MW, Stickland MH, Grant
PJ. Angiotensin converting enzyme (ACE)
insertion/deletion (I/D) polymorphism, and diabetic retinopathy in subjects
with IDDM and NIDDM. Diabet Med 12:997-1001, 1995. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8582133&query_hl=110
389.
390. Lustman PJ, Anderson RJ,
Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a
meta-analytic review of the literature. Diabetes Care 23:934-942, 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10895843&query_hl=114
391.
392. Lustman PJ, Griffith LS, Clouse RE, Freedland
KE, Eisen SA, Rubin EH, Carney RM, McGill JB. Effects
of alprazolam on glucose regulation in diabetes. Results
of double-blind, placebo-contr
393. Maeda S, Haneda M, Guo B, Koya D, Hayashi K,
Sugimoto T, Isshiki K, Yasuda H, Kashiwagi A, Kikkawa R.
Dinucleotide repeat polymorphism of matrix metalloproteinase-9 gene is
associated with diabetic nephropathy. Kidney Int 60:1428-1434, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11576356&query_hl=118
394. Kao YL, Donaghue K, Chan A, Knight J, Silink M.
A novel polymorphism in the aldose reductase gene promoter region is strongly
associated with diabetic retinopathy in adolescents with type 1 diabetes. Diabetes. 1999, 48
(6):1338-40. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10342825&query_hl=120
395. Lajer M, Tarnow L,
Fleckner J, Hansen BV, Edwards DG, Parving HH, Boel E. Association of aldose
reductase gene Z+2 polymorphism with reduced susceptibility to diabetic
nephropathy in Caucasian Type 1 diabetic patients. Diabet Med. 2004, 21:
867-73. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15270790&query_hl=122
396. Taverna MJ, Sola A,
Guyot-Argenton C, Pacher N, Bruzzo F, Chevalier A, Slama G, Reach G, Selam JL.
eNOS4 polymorphism of the endothelial nitric oxide synthase predicts risk for
severe diabetic retinopathy. Diabet Med. 2002 Mar;19(3):240-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11918626&query_hl=124
397. Taverna MJ,
Elgrably F, Selmi H, Selam JL, Slama G. The T-786C and C774T endothelial nitric
oxide synthase gene polymorphisms independently affect the onset pattern of
severe diabetic retinopathy. Nitric Oxide. 2005 May [ E-publication ahead of print] http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15890549&query_hl=126
398. Kao Y, Donaghue KC, Chan A, Bennetts BH, Knight
J, Silink M. Paraoxonase gene cluster is a genetic marker for early
microvascular complications in type 1 diabetes. Diabet Med. 2002, 19: 212-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11918623&query_hl=128
399. Kao YL, Donaghue K, Chan A, Knight J, Silink M.
A variant of paraoxonase (PON1) gene is associated with diabetic retinopathy in
IDDM. J
Clin Endocrinol Metab. 1998 Jul;83(7):2589-92. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9661650&query_hl=120
400. Ray D, Mishra M, Ralph S, Read I, Davies R,
Brenchley P. Association of the VEGF gene with proliferative diabetic
retinopathy but not proteinuria in diabetes. Diabetes.
2004 Mar;53(3):861-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14988276&query_hl=131
401. Taverna MJ, Selam JL, Slama G. Association
between a protein polymorphism in the start codon of the vitamin D receptor
gene and severe diabetic retinopathy in C-peptide-negative type 1 diabetes.
Clin Endocrinol Metab. 2005 May 17; [E-publication ahead of print] http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15899948&query_hl=133
402. Agardh D, Gaur LK, Agardh E, Landin-Olsson M,
Agardh CD, Lernmark A. HLA-DQB1*0201/0302 is associated with severe retinopathy
in patients with IDDM. Diabetologia. 1996, 39: 1313-7 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8932997&query_hl=135
403. Mimura T, Funatsu
H, Uchigata Y, Kitano S, Noma H, Shimizu E, Konno Y, Amano S, Araie M, Yoshino
O, Iwamoto Y, Hori S. Relationship between human leukocyte antigen status and
proliferative diabetic retinopathy in patients with younger-onset type 1
diabetes mellitus. Am J Ophthalmol. 2003, 135: 844-8 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12788125&query_hl=137
404. Agardh E, Gaur LK, Lernmark A, Agardh CD.
HLA-DRB1, -DQA1, and -DQB1 subtypes or ACE gene polymorphisms do not seem to be
risk markers for severe retinopathy in younger Type 1 diabetic patients. J Diabetes Complications. 2004 Jan-Feb;18(1):32-6.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15019597&query_hl=139
405. Wong TY, Cruickshank KJ, Klein R, Klein BE, Moss
SE, Palta M, Riley WJ, Maclaren NK, Vadheim CM, Rotter JI. HLA-DR3
and DR4 and their relation to the incidence and progression of diabetic retinopathy.
Ophthalmology. 2002, 109: 275-81 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11825808&query_hl=141
406. Krolewski AS, Kosinski EJ, Warram JH, Leland OS,
Busick EJ, Asmal AC, Rand LI,
407. Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes.
Am J Public Health 81:1158-1162, 1991.
408. Dorman JS, LaPorte RE, Kuller LH, Cruickshanks
KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL. The
409. Manson JE, Colditz GA, Stampfer MJ, Willett WC,
Krolewski AS, Rosner B, Arky RA, Speizer FE, Hennekens CH. A prospective study of maturity-onset diabetes mellitus and risk of
coronary heart disease and stroke in women. Arch Intern Med
151:1141-1147, 1991.
410. Borch-Johnsen KKreiner S. Proteinuria:
value as predictor of cardiovascular mortality in insulin dependent diabetes
mellitus. Br Med J [Clin Res] 294:1651-1654, 1987.
411. Martin FIHopper JL. The
relationship of acute insulin sensitivity to the progression of vascular
disease in long-term type 1 (insulin-dependent) diabetes mellitus.
Diabetologia 30:149-153, 1987.
412. Orchard TJ, Dorman JS, Maser RE, Becker DJ,
Ellis D, LaPorte RE, Kuller LH, Wolfson SK, Jr., Drash AL. Factors
associated with avoidance of severe complications after 25 yr of IDDM.
413. Orchard TJ. From
diagnosis and classification to complications and therapy. DCCT. Part II? Diabetes Control and
Complications Trial. Diabetes Care 17:326-338,
1994.
414. Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing
RR, Orchard TJ. Coronary artery disease in IDDM.
Gender differences in risk factors but not risk. Arterioscler Thromb Vasc
Biol 16:720-726, 1996. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8640398&query_hl=143
415. Costacou T, Lopes-Virella MF, Zgibor JC, Virella
G, Otvos J, Walsh M, Orchard TJ. Markers of endothelial
dysfunction in the prediction of coronary artery disease in Type 1 diabetes.
The
416. Costacou T, Zgibor
JC, Evans RW, Otvos J, Lopes-Virella MF, Tracy RP, Orchard TJ. The prospective association between
adiponectin and coronary artery disease among individuals with type 1 diabetes. The
417. Crall FV, Jr.Roberts WC. The extramural and intramural coronary
arteries in juvenile diabetes mellitus: analysis of nine necropsy patients aged
19 to 38 years with onset of diabetes before age 15 years. Am J Med
64:221-230, 1978.
418. Valsania P, Zarich SW, Kowalchuk GJ, Kosinski E,
Warram JH, Krolewski AS. Severity of coronary artery
disease in young patients with insulin- dependent diabetes mellitus.
Am Heart J 122:695-700, 1991.
419. Miettinen H, Lehto S, Salomaa V, Mahonen M,
Niemela M, Haffner SM, Pyorala K, Tuomilehto J. Impact
of diabetes on mortality after the first myocardial infarction. The
FINMONICA Myocardial Infarction Register Study Group [see comments].
Diabetes Care 21:69-75, 1998. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9538972&query_hl=149
420. Chun BY, Dobson AJ, Heller RF. The impact of diabetes on survival among patients with first
myocardial infarction. Diabetes Care 20:704-708,
1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9135930&query_hl=151
421. Savage MP,
422. Rozenman Y, Sapoznikov D, Mosseri M, Gilon D,
Lotan C, Nassar H, Weiss AT, Hasin Y, Gotsman MS. Long-term angiographic
follow-up of coronary balloon angioplasty in patients with diabetes mellitus: a
clue to the explanation of the results of the
423. Weintraub WS, Stein B, Kosinski A, Douglas JS,
Jr., Ghazzal ZM, Jones EL, Morris DC, Guyton RA, Craver JM, King SB, III.
Outcome of coronary bypass surgery versus coronary
angioplasty in diabetic patients with multivessel coronary artery disease.
J Am Coll Cardiol 31:10-19, 1998. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9426011&query_hl=156
424. Koistinen MJ. Prevalence
of asymptomatic myocardial ischaemia in diabetic subjects. BMJ
301:92-95, 1990.
425. Orchard TJ, Olson JC, Erbey JR, Williams K,
Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related
factors, but not glycemia, predict coronary artery disease in type 1 diabetes:
10-year follow-up data from the Pittsburgh Epidemiology of Diabetes
Complications Study. Diabetes Care. 2003; 26:
1374–1379 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12716791&query_hl=159
426. Soedamah-Muthu SS, Chaturvedi N, Toeller M,
Ferriss B, Reboldi P, Michel G, Manes C, Fuller JH. EURODIAB
Prospective Complications Study Group. Risk factors for coronary heart
disease in type 1 diabetic patients in
427. Yamasaki Y, Kawamori R, Matsushima H, Nishizawa
H, Kodama M, Kajimoto Y, Morishima T, Kamada T. Atherosclerosis
in carotid artery of young IDDM patients monitored by ultrasound
high-resolution B-mode imaging. Diabetes 43:634-639, 1994.
428. Kanters SD, Algra A, Banga JD. Carotid intima-media thickness in hyperlipidemic type I and type II
diabetic patients. Diabetes Care 20:276-280,
1997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9051371&query_hl=163
429. Nathan DM, Lachin J, Cleary P, Orchard T,
Brillon DJ, Backlund JY, O’Leary DH, Genuth S. Diabetes Control and
Complications Trial; Epidemiology of Diabetes Interventions and Complications
Research Group. Intensive diabetes therapy and carotid intima-media
thickness in type 1 diabetes mellitus. N Engl J Med. 2003; 348:
2294–2303 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12788993&query_hl=165
430. Snell-Bergeon JK, Hokanson JE, Jensen L,
MacKenzie T, Kinney G, Dabelea D, Eckel RH, Ehrlich J, Garg S, Rewers M.
Progression of coronary artery calcification in type 1 diabetes: the importance
of glycemic control. Diabetes Care. 2003 Oct;26(10):2923-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14514603&query_hl=167
431. Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson
JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M; The Coronary Artery
Calcification in Type 1 Diabetes Study. Effect of type 1 diabetes on the gender
difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study.
Diabetes. 2003 Nov;52(11):2833-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14578303&query_hl=169
432. Wadwa RP, Kinney
GL, Maahs DM, Snell-Bergeon J, Hokanson JE, Garg SK, Eckel RH, Rewers M.
Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diabetes Care. 2005 May;28(5):1051-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15855566&query_hl=171
433. Maahs DM, Ogden LG, Kinney GL, Wadwa P,
Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low
plasma adiponectin levels predict progression of coronary artery calcification.
Circulation. 2005 Feb 15;111(6):747-53.
Epub 2005 Feb 7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15699257&query_hl=173
434. Maahs DM, Kinney GL, Wadwa P, Snell-Bergeon JK,
Dabelea D, Hokanson J, Ehrlich J, Garg S, Eckel RH, Rewers MJ. Hypertension
prevalence, awareness, treatment, and control in an adult type 1 diabetes
population and a comparable general population. Diabetes
Care. 2005 Feb;28(2):301-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15677783&query_hl=175
435. Hokanson JE, MacKenzie T, Kinney G,
Snell-Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating changes
in coronary artery calcium: an analytic method that accounts for interscan
variability. AJR Am J Roentgenol. 2004 May;182(5):1327-32. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15100140&query_hl=177
436. Snell-Bergeon JK,
Hokanson JE, Kinney GL, Dabelea D, Ehrlich J, Eckel RH, Ogden L, Rewers
M. Measurement of abdominal fat by
CT compared to waist circumference and BMI in explaining the presence of
coronary calcium. Int J Obes Relat Metab Disord. 2004 Dec;28(12):1594-9.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15467773&query_hl=179
437. Hokanson JE, Cheng S, Snell-Bergeon JK, Fijal
BA, Grow MA, Hung C, Erlich HA, Ehrlich J, Eckel RH, Rewers M. A common
promoter polymorphism in the hepatic lipase gene (LIPC-480C>T) is associated
with an increase in coronary calcification in type 1 diabetes. Diabetes. 2002
Apr;51(4):1208-13. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11916946&query_hl=181
438. Wadwa RP, Rewers M. Update
on noninvasive detection of cardiovascular in diabetes. Curr Opin
Endocrinol Diabetes 2005, 12: 267-272
439. Libby P, Nathan DM, Abraham K, Brunzell JD,
Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S,
Wassef M, Rabadan-Diehl C; National Heart, Lung, and Blood Institute; National
Institute of Diabetes and Digestive and Kidney Diseases Working Group on
Cardiovascular Complications of Type 1 Diabetes Mellitus. Report
of the National Heart, Lung, and Blood Institute-National Institute of Diabetes
and Digestive and Kidney Diseases Working Group on Cardiovascular Complications
of Type 1 Diabetes Mellitus. Circulation. 2005
Jun 28;111(25):3489-93. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15983263&query_hl=189
440. Rewers M, Zimmet P. The rising
tide of childhood type 1 diabetes--what is the elusive environmental trigger?
Lancet.
2004 Nov 6-12;364(9446):1645-7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15530607&query_hl=191
441. Berger B. Stenstrom G. Sundkvist G. Incidence,
prevalence, and mortality of diabetes in a large population. A
report from the Skaraborg Diabetes Registry. Diabetes
Care. 22(5):773-8, 1999 May. 504. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10332680&query_hl=193
442. Wu D, Kendall D, Lunt H, Willis J, Darlow B,
Frampton C. Prevalence of Type 1 diabetes in New Zealanders aged 0-24
years. N Z Med J. 2005 Jul 15;118(1218):U1557. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16027748&query_hl=195
443. Urbonaite B, Zalinkevicius R, Green A.
Incidence, prevalence, and mortality of insulin-dependent (type 1) diabetes
mellitus in Lithuanian children during 1983-98. Pediatr Diabetes. 2002 Mar;3(1):23-30
444. Motala AA. Diabetes trends in
445. Samuelsson U, Sadauskaite V, Padaiga Z,
Ludvigsson J; DEBS Study Group. A fourfold difference in the
incidence of type 1 diabetes between
446. Macedo A, Jorge Z, Lacerda Nobre E, Pratas S,
Jacome de Castro J. Prevalence of Type 1 diabetes mellitus in
447. Casu A, Pascutto C, Bernardinelli L, Songini M.Type 1 diabetes among Sardinian children is
increasing: the Sardinian diabetes register for children aged 0-14 years
(1989-1999). Diabetes Care. 2004 Jul;27(7):1623-9.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15220238&query_hl=203
448. Zhao HX, Stenhouse E, Sanderson E, et al.
Continued rising trend of childhood Type 1 diabetes mellitus in Devon and
Cornwall, England Diab Med 20 (2): 168-170 FEB 2003 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12581273&query_hl=205
449. Feltbower RG,
Bodansky HJ, McKinney PA, et al. Trends
in the incidence of childhood diabetes in south Asians and other children in
Bradford, UK Diab Med 19 (2): 162-166 FEB 2002 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11874434&query_hl=207
450. Lee WR.The changing demography
of diabetes mellitus in
451. Joner, G, Stene L., Sovik O., the Norwegian
Childhood Diabetes Study Group Nationwide, Prospective Registration of Type 1
Diabetes in Children Aged <15 Years in Norway 1989-1998: No increase but
significant regional variation in incidence. Diabetes Care 2004, 27(7):
1618-1622 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15220237&query_hl=213
452. Kyvik KO, Nystrom L, Gorus F, Songini M, Oestman
J, Castell C, Green A, Guyrus E, Ionescu-Tirgoviste C,
453. Pundziute-Lycka A,
Dahlquist G, Urbonaite B, Zalinkevicius R; Swedish Childhood Diabetes Study
Group; Lithuanian Childhood Diabetes Study Group. Time trend of childhood type 1 diabetes incidence in
454. Green A, Patterson CC; EURODIAB TIGER Study
Group.
455. Cotellessa M, Barbieri P, Mazzella M, Bonassi S,
Minicucci L, Lorini R. High incidence of childhood type 1 diabetes in
456. Bruno G, Cerutti F, Merletti F, Cavallo-Perin P,
Gandolfo E, Rivetti M, Runzo C, Pinach S, Pagano G; Piedmont Study Group for
Diabetes Epidemiology. Residual beta-cell function and male/female ratio are
higher in incident young adults than in children: the registry of type 1
diabetes of the
457. Carle F, Gesuita R,
Bruno G, Coppa GV, Falorni A, Lorini R, Martinucci ME, Pozzilli P, Prisco F,
Songini M, Tenconi MT, Cherubini V; RIDI Study Group. Diabetes incidence in 0- to 14-year age-group in
458. Newhook LA, Curtis J, Hagerty D, Grant M,
Paterson AD, Crummel C, Bridger T, Parfrey P. High incidence of childhood type
1 diabetes in the Avalon Peninsula, Newfoundland, Canada. Diabetes
Care. 2004 Apr;27(4):885-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15047643&query_hl=227
459.
460. Steck AK, Barriga KJ, Emery LM, Fiallo-Scharer
RV, Gottlieb PA, Rewers MJ. Secondary attack rate of
type 1 diabetes in
461. Rewers M, Zimmet P. The rising
tide of childhood type 1 diabetes--what is the elusive environmental trigger?
Lancet. 2004 Nov 6-12;364(9446):1645-7.
Prevalence of type 1 diabetes http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15530607&query_hl=191
462. Haynes A, Bower C, Bulsara MK, Jones TW, Davis
EA. Continued increase in the incidence of childhood Type 1 diabetes in a
population-based Australian sample (1985-2002). Diabetologia. 2004
May;47(5):866-70 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15095039&query_hl=234
463. Lipman TH, Chang Y, Murphy KM. The epidemiology
of type 1 diabetes in children in
464. Oeltmann JE, Liese
AD, Heinze HJ, Addy CL, Mayer-Davis EJ. Prevalence of diagnosed diabetes among
African-American and non-Hispanic white youth, 1999. Diabetes Care. 2003 Sep;26(9):2531-5 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12941714&query_hl=238
465. Dziatkowiak H, Ciechanowska M, Wasikowa R,
Symonides-lawecka A, Bieniasz J, Trippenbach-Dulska H, Korniszewski L,
Szybinski Z. Increase in the incidence of type 1 diabetes mellitus in children
in three cities in Poland, 1987-1999. J Pediatr Endocrinol
Metab. 2002 Sep-Oct;15(8):1153-60. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12387513&query_hl=240
466. Cepedano Dans A,
Barreiro Conde J, Pombo Arias M; Grupo de Diabetes Infantil de Galicia. Incidence and clinical manifestations at onset of
type 1 diabetes mellitus in
467. Patterson CC, Dahlquist G, Soltesz G, Green A;
EURODIAB ACE Study Group.
468. Feltbower RG,
469. Kretowski A, Kowalska I, Peczynska J, Urban M,
Green A, Kinalska I. The large increase in incidence of Type I diabetes
mellitus in
470. Peczynska J, Urban M, Florys B. The epidemiology of type 1 diabetes in children and adolescents in
north-east
471. Cinek O, Sumnik Z, Vavrinec J.Childhood diabetes
in the
472. Dabelea D on behalf of SEARCH Study Group.The
SEARCH for diabetes in youth study group. Abstracts of
473. Libman IM, Pietropaolo M., Arslanian SA, LaPorte
RE, Becker DJ. Evidence for Heterogeneous Pathogenesis of
Insulin-Treated Diabetes in Black and White Children. Diabetes Care,
October 1, 2003; 26(10): 2876 - 2882. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14514595&query_hl=257
474. Gong CX, Zhu C, Yan C, Liang JP, Ni GC, Gao J,
Li YC, Liu M, Peng XX, Yang Z. Survey of type 1 diabetes incidence in children
from 1997 to 2000 in
475. Charkaluk ML, Czernichow P, Levy-Marchal C.
Incidence data of childhood-onset type I diabetes in
476. Rosenbauer J, Icks A, Schmitter D, Giani G. Incidence of childhood Type I diabetes mellitus is
increasing at all ages in
477. Arpi ML, Fichera G,
Mancuso M, Lucenti C, Italia S, Tomaselli L, Motta RM, Mazza A, Vigneri R,
Purrello F, Squatrito S. A ten-year (1989-1998) perspective study of the
incidence of Type 1 diabetes http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12035936&query_hl=265
478. Kulaylat NA, Narchi H. A twelve year study of
the incidence of childhood type 1 diabetes mellitus in the Eastern Province of
Saudi Arabia. J Pediatr Endocrinol Metab. 2000 Feb;13(2): 135-40. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10711657&query_hl=267
479. Shaltout AA, Moussa
MA, Qabazard M, Abdella N, Karvonen M, Al-Khawari M, Al-Arouj M, Al-Nakhi A,
Tuomilehto J, El-Gammal A; Kuwait Diabetes Study Group. Further evidence for the rising
incidence of childhood Type 1 diabetes in
480. Willis JA, Scott
RS, Darlow BA, Nesbit JW, Anderson P, Moore MP, Lunt H, Cole DR. Incidence of type 1 diabetes mellitus diagnosed
before age 20 years in Canterbury, New Zealand over the last 30 years. J Pediatr Endocrinol Metab. 2002 May;15(5):637-43.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12014523&query_hl=272
481. Mamoulakis D, Galanakis E, Bicouvarakis S,
Paraskakis E, Sbyrakis S. Epidemiology of childhood
type I diabetes in
482. Unachak K, Tuchinda C. Incidence of type 1
diabetes in children under 15 years in northern
483. Pilecki O, Robak-Kontna K, Jasinski D, Bogun-Reszczynska
Z, Bojko-Zbikowska M. Epidemiology of type 1 diabetes mellitus in
484. Martinucci ME, Curradi G, Fasulo A, Medici A,
Toni S, Osovik G, Lapistkaya E, Sherbitskaya E. Incidence of childhood type 1
diabetes mellitus in Gomel, Belarus. J Pediatr Endocrinol Metab. 2002 Jan;15(1):53-7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11822581&query_hl=280
485. Waldhor T, Schober E, Karimian-Teherani D, Rami
B; Austrian Diabetes Incidence Study Group. Regional differences and temporal
incidence trend of Type I diabetes mellitus in
486. van Wouwe JP, Mattiazzo GF, el
Mokadem N, Reeser HM, Hirasing RA.
The incidence and initial symptoms of diabetes mellitus type 1 in
0-14-year-olds in the
487. Roche EF, Menon A, Gill D, Hoey HM. Incidence of
type 1 diabetes mellitis in children aged under 15
years in the
488. Ogle GD, Lesley J, Sine P, McMaster P. Type 1
diabetes mellitus in children in Papua New Guinea.P N G Med J. 2001
Sep-Dec;44(3-4):96-100. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12422979&query_hl=289
489. Lopez-Siguero JP,
Del Pino-De la Fuente A, Martinez-Aedo MJ, Moreno-Molina JA. Increased incidence of type 1
diabetes in the south of
490. Ionescu-Tirgoviste C, Guja C, Calin A, Mota M.
Related An increasing trend in the incidence of type 1 diabetes mellitus in
children aged 0-14 years in Romania--ten years (1988-1997) EURODIAB study
experience. J Pediatr Endocrinol Metab. 2004 Jul;17(7):983-91. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15301046&query_hl=293
491. Lipton R, Keenan H, Onyemere KU, Freels S.
Incidence and onset features of diabetes in African-American and Latino
children in
492. Rytkonen M, Moltchanova E, Ranta J, Taskinen O,
Tuomilehto J, Karvonen M; SPAT Study Group; Finnish Childhood Diabetes Registry
Group. The incidence of type 1 diabetes among children in
493. Michalkova D, Minarik P, Hlava P, Camajova J,
Nazarov V; Slovak Epidemiological Study Group of Children Diabetologists.
Trends in the incidence of childhood-onset type 1 diabetes
in
494. Samuelsson U, Lofman O. Geographical mapping of
type 1 diabetes in children and adolescents in south east Sweden. J Epidemiol Community Health. 2004 May;58(5):388-92.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15082736&query_hl=301
495. Kondrashova A, Reunanen A, Romanov A, Karvonen
A, Viskari H, Vesikari T, Ilonen J, Knip M, Hyoty H. A
six-fold gradient in the incidence of type 1 diabetes at the eastern border of
496. Podar T, Solntsev
A, Karvonen M, Padaiga Z, Brigis G, Urbonaite B, Viik-Kajander M, Reunanen A,
Tuomilehto J. Increasing incidence of childhood-onset type I diabetes in 3
Baltic countries and Finland 1983-1998. Diabetologia. 2001 Oct;44 Suppl 3:B17-20. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11724410&query_hl=305
497. Weets I, De Leeuw IH, Du Caju MV, Rooman R,
Keymeulen B, Mathieu C, Rottiers R, Daubresse JC, Rocour-Brumioul D, Pipeleers
DG, Gorus FK; Belgian Diabetes Registry. The incidence of type 1 diabetes in
the age group 0-39 years has not increased in
498. Israel IDDM Registry Study Group. Incidence of IDDM between the ages 0-17 years in
499. Gyurus E, Green A, Patterson CC, Soltesz G;
Hungarian Childhood Diabetes Epidemiology Study Group. Dynamic
changes in the trends in incidence of type 1 diabetes in children in
500. Kadiki OA, Roaeid RB. Incidence of type 1 diabetes in
children (0-14 years) in
501. Bratina NU, Tahirovic H, Battelino T, Krzisnik
C. Incidence of childhood-onset Type I diabetes in
502. Schoenle EJ, Lang-Muritano M, Gschwend S,
Laimbacher J, Mullis PE, Torresani T, Biason-Lauber A, Molinari L. Epidemiology
of type I diabetes mellitus in Switzerland: steep rise in incidence in under 5
year old children in the past decade. Diabetologia. 2001 Mar;44(3):286-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11317657&query_hl=322
503. Raymond NT, Jones
JR, Swift PG, Davies MJ, Lawrence G, McNally PG, Burden ML, Gregory R, Burden
AC, Botha JL. Comparative incidence of
Type I diabetes in children aged under 15 years from South Asian and White or
Other ethnic backgrounds in Leicestershire, UK, 1989 to 1998.Diabetologia. 2001
Oct;44 Suppl 3:B32-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11724414&query_hl=324
504. Lora-Gomez RE, Morales-Perez FM, Arroyo-Diez FJ,
Barquero-Romero J. Incidence of Type 1 diabetes in children in Caceres, Spain,
during 1988-1999. Diabetes Res Clin Pract. 2005 Aug;69(2):169-74. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16005366&query_hl=326
506. Carrasco E,
Perez-Bravo F, Dorman J, Mondragon A, Santos JL. Increasing incidence of type 1 diabetes in population
from Santiago of Chile: trends in a period of 18 years (1986-2003). Diabetes Metab Res Rev. 2005 May 13; [Epub ahead of
print] http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15892034&query_hl=330
507. Pishdad GR. Low incidence of type 1 diabetes in
508. Svensson J, Carstensen B, Molbak A, Christau B,
Mortensen HB, Nerup J, Borch-Johnsen K: Increased risk of childhood type 1
diabetes in children born after 1985. Diabetes Care 2002, 25:2197–2201 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12453960&query_hl=335
509. Jarosz-Chobot P, Otto-Buczkowska E, Koehler B,
Matlakiewicz E, Green A. Increased trend of type 1 diabetes mellitus in
children's population (0-14 years) in Upper Silesia region (
510. Tzaneva V, Iotova V, Yotov Y. Significant
urban/rural differences in the incidence of type 1 (insulin-dependent) diabetes
mellitus among Bulgarian children (1982-1998). Pediatr Diabetes. 2001 Sep;2(3):103-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15016192&query_hl=339
512. Mooney JA, Helms PJ, Jolliffe IT, Smail P;
Scottish Study Group for the Care of Diabetes in the Young. Seasonality of type
1 diabetes mellitus in children and its modification by weekends and holidays:
retrospective observational study. Arch Dis Child.
2004 Oct;89(10):970-3 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15383444&query_hl=343
513. Collado-Mesa F, Diaz-Diaz O, Ashkenazi I,
Seasonality of birth and Type 1 diabetes onset in children (0-14 years) in
Cuba. Diabet Med. 2001 Nov;18(11):939-40. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11703443&query_hl=345
514. Oeltmann JE, Liese AD, Heinze
HJ, Addy CL, Mayer-Davis EJ. Prevalence of diagnosed diabetes among African-American and
non-Hispanic white youth, 1999. Diabetes Care. 2003
Sep;26(9):2531-5 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12941714&query_hl=347
515. Rosenbauer J, Icks A, Giani G. Incidence and
prevalence of childhood type 1 diabetes mellitus in
516. Harvey JN. Craney L. Kelly D. Estimation of the
prevalence of diagnosed diabetes from primary care and secondary care source
data: comparison of record linkage with capture-recapture analysis. Journal of Epidemiology & Community Health. 56(1):18-23,
2002 Jan. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11801615&query_hl=351
517. Giralt Muina P,
Santillana Ferrer L, Madrigal Barchino D, Merlo Garrido A, Toledo De La Torre
B, Anaya Barea F. Incidence of diabetes
mellitus and prevalence of type 1A diabetes mellitus in children younger than
16 years old from the province of Ciudad Real. Anales Espanoles de Pediatria. 55(3):213-8,
2001Sep. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11676895&query_hl=353
518. Blanchard JF. Dean H. Anderson K. Wajda A.
Ludwig S. Depew N. Incidence and prevalence of diabetes in children aged 0-14
years in
519. Virtanen
SM, Kenward MG, Erkkola M, Kautiainen S, Kronberg-Kippilä C, Hakulinen T,
Ahonen S, Uusitalo L, Niinistö S, Veijola R, Simell O, Ilonen J, Knip M. Age at
introduction of new foods and advanced beta cell autoimmunity in young children
with HLA-conferred susceptibility to type 1 diabetes. Diabetologia.
2006 Jul;49(7):1512-21. Epub 2006 Apr 5.
http://www.ncbi.nlm.nih.gov/pubmed/16596359
520. Scott FW, Rowsell P,
Wang GS, Burghardt K, Kolb H, Flohé S. Oral exposure
to diabetes-promoting food or immunomodulators in neonates alters gut cytokines
and diabetes. Diabetes. 2002 Jan;51(1):73-8.
http://www.ncbi.nlm.nih.gov/pubmed/11756325
521. MacFarlane AJ,
Burghardt KM, Kelly J, Simell T, Simell O, Altosaar I, Scott FW. A type 1 diabetes-related protein from wheat (Triticum aestivum).
cDNA clone of a wheat storage globulin, Glb1, linked
to islet damage. J Biol Chem. 2003 Jan 3;278(1):54-63.
Epub 2002 Oct 29.
http://www.ncbi.nlm.nih.gov/pubmed/12409286
522. Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia. 1999 Jan;42(1):51-4.
http://www.ncbi.nlm.nih.gov/pubmed
523. Norris JM, Yin X, Lamb
MM, Barriga K, Seifert J, Hoffman M, Orton HD, Barón AE, Clare-Salzler M, Chase
HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M. Omega-3 polyunsaturated fatty
acid intake and islet autoimmunity in children at increased risk for type 1
diabetes. JAMA. 2007 Sep 26;298(12):1420-8.
http://www.ncbi.nlm.nih.gov/pubmed/17895458
524. Lamb MM, Yin X, Zerbe GO, Klingensmith GJ, Dabelea D, Fingerlin TE,
Rewers M, Norris JM. Height growth
velocity, islet autoimmunity and type 1 diabetes development: the Diabetes Autoimmunity
Study in the Young. Diabetologia. 2009 Oct;52(10):2064-71. Epub 2009 Jun 23.
http://www.ncbi.nlm.nih.gov/pubmed/19547949
525. Ivarsson A, Hernell O, Stenlund H, Persson LA. Breast-feeding protects against celiac disease.
Am J Clin Nutr. 2002
May;75(5):914-21.
http://www.ncbi.nlm.nih.gov/pubmed/11976167