9/13/12
Chapter 8
Autoimmune Polyendocrine Syndromes
J. M. Barker and G. S. Eisenbarth
Examples of Polyendocrine Autoimmune Syndromes include:
1. Autoimmune polyglandular syndrome type I1 2, 3(APS-1, APECED: autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, MIM number 240300[Online Mendelian Inheritance in Man)
2. The autoimmune polyendocrine syndrome type II (APS-2, Schmidt’s syndrome MIM number 269200) 4-6 7
3. IPEX (Immunodysregulation, Polyendocrinopathy and Enteropathy, X-Linked), also termed XLAAD X-Linked Autoimmunity Allergic-Dysregulation Syndrome or XPID X-Linked Polyendocrinopathy, Immune Dysfunction and Diarrhea, MIM number 304790 and 300292)
4. Non-organ-specific autoimmunity (e.g., lupus erythematosus) associated with anti-insulin receptor antibodies8, 9
5. Thymic tumors with associated endocrinopathy10-13, 13
6. Graves’ disease associated with insulin autoimmune syndrome14, 15
7. POEMS syndrome (Polyneuropathy, Organomegaly, Endocrinopathy, M-spike, Skin changes MIM 192240)16, 17
8. Congenital rubella infection followed by development of thyroiditis and type 1 diabetes +/- other autoimmune disorders.
9. Anti-Pit1 Antibody: Adult combined GH, prolactin and TSH deficiency.
The diverse names given to the polyendocrine autoimmune syndromes reflect the large number of studies and case reports concerning these patients and heterogeneity in their clinical presentation. The two major autoimmune endocrine syndromes, APS-1 and APS-2, both have Addison’s disease as a prominent component. The major autoimmune polyendocrine syndromes have a strong genetic component with the type 2 syndrome occurring in multiple generations and the type I syndrome in siblings.
The major illnesses associated with both APS-1 and APS-2 are listed in Table 8.1 and differences between the syndromes are outlined in Table 8.2. Knowledge of disease associations and inheritance pattern facilitates early diagnosis of component illnesses7. Patients with APS-1 and APS-2 develop multiple diseases over time and approximately one out of seven relatives of such patients have an undiagnosed autoimmune disorder (most often hypothyroidism for the type 2 syndrome)18. The individual polyendocrine autoimmune syndromes, their immunogenetics, pathogenesis and selected aspects of therapy will be reviewed in this chapter.
Autoimmune Polyendocrine Syndrome Type 1 (APS-1)
Major components of the APS-1 autoimmune polyendocrine syndrome (also termed APECED autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy 19-22 include hypoparathyroidism23-25, mucocutaneous candidiasis26-28, and Addison’s disease (Table 8.1). Other diseases associated with APS-1 include autoimmune hepatitis29 primary hypothyroidism, a malabsorption syndrome30, 31, vitiligo, pernicious anemia32, type 1 diabetes, alopecia33, primary hypogonadism34, cutaneous abnormalities1, pulmonary disease35, 36, ovarian failure37, 38, pericarditis39, cerebellar degeneration40, encephalopathy41, asplenia42, esophageal cancer43, polyneuropathy44, pure red cell aplasia45, 46, etc. Of note, in a study by Friedman and coworkers47 four of nine patients with APS-1 were asplenic.
An unusual manifestation of the disorder is the development of refractory diarrhea/obstipation that may be related to “autoimmune” destruction of enterochromaffin or enterochromaffin like cells, associated with specific autoantibodies48-50. Kampe and coworkers have reported that antibodies to Histidine Decarboxylase are associated with a history of intestinal dysfunction51 and multiple reports document loss of gastric and intestinal endocrine cells 51 including chromogranin A and serotonin producing cells50. A number of rare manifestations of APS-1 are particularly troublesome. A number of patients have been reported with severe lung disease, often leading to death. The lung disorders have been described as autoimmune bronchiolitis, bronchiolitis obliterans organizing pneumonia, chronic hypersensitivity pneumonitis19, 35, 52. Patients are at risk for developing esophageal and oral cancers presumably related to chronic candida infections43.
The onset of the disease is usually in infancy. Chronic mucocutaneous candidiasis is often the first disease detected, followed by the later development of adrenal insufficiency. The etiology of the mucocutaneous candidiasis in the absence of systemic candidiasis in the APS-1 syndrome has been related to anti-cytokine autoantibodies (anti-IL17A, IL17F and IL22) related to Th17 T cells 53 and depressed production of IL17F and IL22 by peripheral blood mononuclear cells 54 through increased IL17 with decreased IL22 also reported26. Decades may elapse between the diagnosis of one disease and another in the same individual and the order of disease appearance is not invariant. There is a remarkably strong association with autoantibodies targeting interferon alpha and omega which are “diagnostic” except for the additional association with thymomas with such autoantibodies13, 55.
The syndrome is almost always inherited in an autosomal recessive manner linked to mutation of the AIRE gene 56 57(AIRE: Autoimmune Regulator gene) on chromosome 2158; 59. The immunodeficiency underlying disease susceptibility is secondary to autosomal recessive mutations of this transcription factor60. Studies of autoimmune disorders including Addison’s disease, but in patients without the APS-1 syndrome, indicate that AIRE mutations are not involved in these more common diseases61. In contrast, there is evidence that in rare diseases with abnormal T cell development (e.g. T-B-SCID, 0menn syndrome (OM1M 603 554) there is abnormal thymocyte epithelial interaction and deficient thymic AIRE and lack of expression of AIRE dependent “peripheral” molecules such as insulin 62. There is no association of the overall syndrome with specific HLA class II alleles, though there is increasing evidence that specific HLA alleles determine the probability of the specific organs targeted by an individual63. In particular DQB1*0602 appears to protect from type 1 diabetes and DR3 increase the risk of type 1 diabetes in patients with the APS-1 syndrome, as it does for the common variety of type 1A diabetes.
The knockout of the AIRE gene by two groups in mouse models indicates that mice lacking AIRE develop widespread but clinically mild autoimmunity. In particular autoantibodies reacting with multiple organs and T cell infiltrates of multiple organs are observed 64, 65. A potentially very important finding is a decrease in expression within the thymus of what have been termed “peripheral antigens”65. Hanahan and coworkers coined the term peripheral antigen expressing cells, for cells within the thymus that express “Peripheral” molecules such as insulin66.
It is now apparent that multiple such molecules are expressed within subsets of cells within the thymus, with relatively few cells expressing any given molecule, but many thymic cells expressing multiple different “peripheral antigens” 67-72. It is likely that the cell types expressing these molecules are primarily medullary thymic epithelial cells and cells of the macrophage, dendritic lineage. For instance, with loss of AIRE gene function, insulin message73 disappears from the thymus65. Altering insulin expression in the thymus (e.g. insulin 2 gene knockout in NOD mouse) can have a dramatic effect on development of autoimmunity 74, 74-78 and the protection afforded by the insulin gene 5’VNTR in man is associated with greater thymic insulin message 68, 79. Thus an attractive hypothesis is that mutations of the AIRE gene (e.g. APS-1 syndrome) cause loss of peripheral antigen expression in the thymus80 and probably decreased deletion of autoreactive T lymphocytes that target such peripheral antigens. Of note, peripheral antigens are also expressed within other lymphoid organs81 and AIRE is reported to be functionally relevant in lymph nodes82, 83.
A single family has been described with an autosomal dominant form of the disease (thyroiditis prominent in this family)84, and of note Anderson and colleagues have produced an animal model of the dominant mutation found in this family57. The specific knockin mutation (G228W) of AIRE functioned as a dominant negative, recruiting wild type AIRE away from active sites of transcription, decreased thymic messenger RNA for multiple AIRE regulated thymic genes, and the mice develop lachrymal, salivary, and thyroid infiltrates and progressive peripheral neuropathy. Of note with the mutation on an NOD background diabetes is not accelerated and the mice do not develop pancreatitis (found in NOD mice with homozygous knockouts of AIRE). AIRE presumably acts by altering transcription of multiple thymic genes and has important interaction with chromatin57.
|
Table 8.1. Disease Associations |
|
|
|
Autoimmune Polyendocrine Syndrome Autoimmune Polyendocrine Syndrome |
|
Type 1 Incidence Type 2 Incidence |
|
|
|
Mucocutaneous candidiasis1, 85 C* Addison’s disease86 C |
|
Addison’s disease1, 85 C |
|
Hypoparathyroidism85 C |
|
Chronic active hepatitis1, 85, 87 C |
|
Graves’ disease I Graves’ disease I |
|
Autoimmune thyroiditis85, 88 I Autoimmune thyroiditis89 I |
|
Pernicious anemia C Pernicious anemia90, 91 C |
|
Vitiligo C Vitiligo90, 92-94 C |
|
Type 1 DM (18%) 1, 85 I Type 1 DM (50%) C |
|
Alopecia33, 95, 96 I Alopecia I |
|
Myasthenia gravis97, 98 I |
|
Malabsorption syndrome30, 85 I Celiac disease, Dermatitis C |
|
herpetiformis99-101 |
|
IgA deficiency I IgA deficiency94 I |
|
Serositis102 R |
|
Asplenism47 C |
|
Ectodermal dysplasia1 C |
|
Keratitis103 C |
|
Hypogonadism34, 85 C Hypogonadism104 I |
|
Stiff-man syndrome105 R |
|
Parkinsons disease I |
|
Dental enamel and nail dystrophy1 C |
|
Idiopathic heart block90 R |
|
Pure red cell aplasia45 R |
|
Idiopathic thrombocytopenia106, 107 I |
|
Hypophysitis108, 109 R |
|
Autoimmune Bronchiolitis110 R |
|
*C=common within syndrome; R=rare; I=intermediate |
Patients with APS-1 express autoantibodies reacting with a remarkable diversity of autoantigens, both autoantibodies specifically only detected in APS-1 patients and autoantibodies found in common isolated examples of the autoimmune disorder (e.g. 21 hydroxylase autoantibodies for Addison’s disease). Kampe and coworkers have recently described the presence of autoantibodies reacting with NALP5 in approximately 50% of patients with APS-1 and hypoparathyroidism, but not found in patients with isolated hypoparathyroidism25. The NALP5 gene is specifically expressed in the parathyroid and ovary. Prospective studies are needed to define when autoantibodies to NALP5 appear and whether they disappear following the development of hypoparathyroidism in a subset of patients, thus correlating with lack of the autoantibodies in approximately 50% of the APS-1 patients with hypoparathyroidism. Why NALP5 should be highly expressed in the parathyroid glands and why they are such a prominent target of autoantibodies in this particular syndrome are unknown. The molecule NALP5 (NACHT leucine-rich-repeat protein 5) is a member of a family of molecules important for activating the innate immune system as part of the inflammasome pathway and are potent activators of interleukin 1111-113. They are involved in processes as diverse as autoinflammatory diseases, the inflammation secondary to the sensing of urate crystals in gout114, and even (NALP3) the adjuvant effects of alum115. A polymorphism of NALP1 has been associated with vitiligo116.
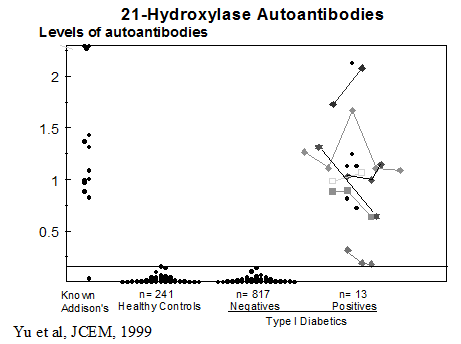
Particular patterns of autoantibodies are associated with APS-1117-119. The presence of anti-adrenal autoantibodies (e.g. 21-hydroxylase) is strongly associated with subsequent development of Addison’s disease. In addition, anti-GAD autoantibodies of patients with the APS I syndrome differ from anti-GAD autoantibodies of typical patients with type 1 diabetes in terms of being able to react with GAD on Western blots and inhibit enzymatic activity. A minority (18%) of patients with APS I develop type 1 diabetes85. Many patients express ICA and anti-GAD autoantibodies [“restricted” ICA (41%)] with relatively little progression to diabetes. Patients expressing multiple anti-islet autoantibodies are at higher risk for progression to diabetes. APS-1 patients express additional autoantibodies consistent with widespread loss of tolerance to multiple self antigens120. A study by Soderbergh and coworkers have analyzed the prevalence of a series of autoantibodies in patients with APS-1121 with for instance IA-2 autoantibodies highly associated with type 1 diabetes, while GAD autoantibodies were more associated with intestinal dysfunction. Hypogonadism was associated with side-chain cleavage enzyme (SCC) while Addison’s disease was associated with both SCC autoantibodies and 21-hydroxylase autoantibodies.
|
Table 8.2. Comparison of APS-1 and APS-2
|
|
APS-I APS-2
|
|
Onset infancy Older onset Siblings affected (autosomal recessive, Multiple generations Chromosome 21, AIRE gene) Not HLA associated but specific disease DR3/DR4 associated Autoantibodies to Type 1 Interferons and Th17 cytokines (IL17A, IL17F, IL22) No autoantibodies to cytokines Asplenism Mucocutaneous candidiasis No defined immunodeficiency |
Diagnosis
The diagnosis of APS-1 is usually made with finding 2 or 3 of the following: mucocutaneous candidiasis, hypoparathyroidism and/or adrenal insufficiency (or autoantibodies against CYP450c21, 21 hydroxylase). It has recently been reported that 100% of patients with APS-1 express autoantibodies reacting with interferon-omega and the great majority express autoantibodies reacting with interferon alpha55, 122. We have developed an ELISA format assay utilizing time resolved fluorescence of Europium to detect autoantibodies and can confirm the very high prevalence of anti-interferon autoantibodies in patients with APS-1122. A commercial hit for such autoantibodies is als abailable from RSR/Kronus. The autoantibodies are apparently the first autoantibodies to develop and remain throughout the disease course, a very remarkable finding. Thus an initial diagnostic screen can be performed for autoantibodies reacting with interferons (a subset of patients with myasthenia gravis and thymoma as well as patients treated with interferons also produce anti-interferon antibodies). Final diagnosis is usually dependent upon direct AIRE gene sequencing, with difficulty arising in defining mutations on both genes in the presence of deletions.
Given that the individual components of disease develop over years to decades, one must be vigilant for other associated autoimmune disorders. Perheentupa recommends that individuals under the age of 30 years with any of the following: chronic or recurring mucocutaneous candidiasis, hypoparathyroidism, adrenal insufficiency, chronic gastrointestinal disease characterized by obstipation, diarrhea or steatorrhea, alopecia, vitiligo, noninfectious hepatitis, keratoconjunctivitis or urticaria-like erythema with fever should be closely evaluated for signs of ectodermal dysplasia and consideration for AIRE gene mutational analysis entertained. If more than one of these components is identified, the individuals should be closely followed for the development of additional disease. Siblings of affected individuals need only have one of the above to warrant more aggressive surveillance 123. Of note, multiple mutations of the AIRE gene have been implicated in the pathogenesis of disease. Therefore, the sensitivity of mutational analysis is dependent upon the number of mutations screened for and the underlying prevalence of the mutations in the population 56.
Surveillance
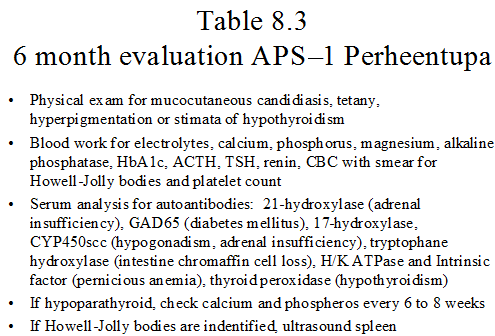
Once the diagnosis of APS-1 is established, the individuals should be referred to a center with experience following these patients and enrolled in a systematic screening regimen with the goal of identifying developing autoimmune disease prior to significant clinical sequelae are reached. Ideally, these individuals should be evaluated every six months for maintenance of their current endocrine disorders and surveillance for future disorders. Table 8.3 outlines recommended screening tests. Mucocutaneous candidiasis may manifest itself anywhere in the GI tract. Therefore, the organism may be identified from the oral mucosa or stool smears. Individuals with symptoms of dysphasia or chest pain should be evaluated by endoscopy to identify strictures. Of note, sudden hypercalcemia in hypoparathyroid individuals may mark the beginning of adrenal insufficiency and deserves evaluation 124. The gastrointestinal system may be involved (Table 8.4) 48, 49, 123. Symptoms of diarrhea, malabsorption with failure to thrive in children and/or obstipation may be identified. These symptoms maybe due to the underlying endocrine disease (e.g. diarrhea with the hypocalcemia of hypoparathyroidism) or may be a manifestation of a new disorder. Evaluation of these symptoms may require investigation for other autoimmune GI disorders such as pernicious anemia and celiac disease, evaluation for fat malabsorption that may be observed with exocrine pancreatic insufficiency and consideration of an autoantibody to endocrine cells of the GI tract (intestinal biopsy often reveals loss of enteroendocrine cells including serotonin expressing cells50), and close consultation with a gastroenterologist is needed. Monitoring for asplenism which can develop over time is also important 42.Table 8.5 lists several conditions that have been rarely identified in individuals with APS-1123.


Treatment
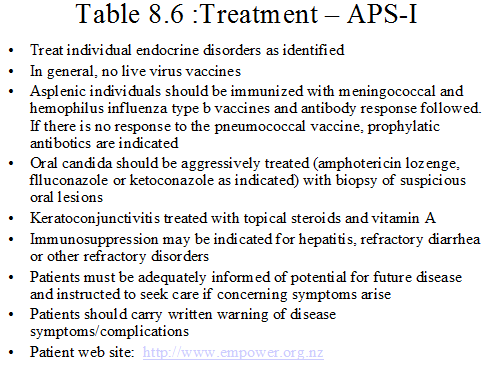
Patient education is a critical element of a successful treatment plan. These individuals often suffer from multiple endocrinopathies and are at risk for the development of further disease. They must be aware of signs and symptoms of new disease and carry with them information about their disease, should emergency care be needed 123. Treatment will in part depend upon the autoimmune disorder identified (table 8.6). Of note, aggressive therapy of oral candidiasis 125 is indicated in an effort to prevent the late complication of epithelial carcinoma. Any lesions that are suspicious should be biopsied 123. Keratoconjunctivitis must also be aggressively treated to prevent a decrease in visual acuity 103. Asplenic individuals are also at risk for fulminant sepsis 126. They must be aggressively identified and immunized for hemophilus influenza, meningococcus and pneumococcus. If the individual mounts an inadequate antibody response, daily antibiotics are indicated, as prophylaxis and emergency care should be sought for fever (table 8.6).
Autoimmune Polyendocrine Syndrome Type 2 (APS-2, Schmidt’s Syndrome)
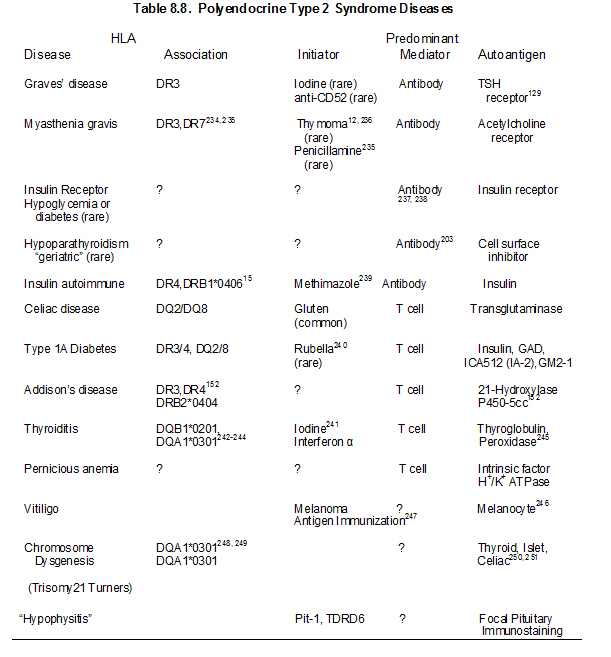
The type 2 syndrome is the most common autoimmune polyendocrine syndrome127. In 1926, Schmidt described two subjects with thyroiditis and Addison’s disease4. Other diseases of the APS 2 include Graves’ disease (thyrotoxicosis), primary hypothyroidism89, insulin-dependent or type 1A diabetes mellitus (IDDM), celiac disease101, 128-130 vitiligo90, 92-94 serositis102, IgA deficiency94, primary hypogonadism104, stiff-man syndrome105, alopecia, pernicious anemia90, 91, myasthenia gravis97, hypopituitarism131, 132 and Parkinson’s disease. Organ-specific autoantibodies in the absence of overt disease is also frequently present in patients and their relatives133. Some authors divide the APS-2 syndrome based upon the specific disease components reserving APS-2 for Addison’s disease plus autoimmune thyroid disease or type 1 diabetes (e.g. APS-3 for thyroid autoimmunity plus other autoimmune (not Addison’s or hypoparathyroidism); APS-4 for two or more other organ specific autoimmune diseases). In that the additional divisions at present provide limited prognostic information (e.g. patient with diabetes and thyroiditis are at risk for Addison’s) we will use APS-2 as inclusive of multiple autoimmune disorders with one or more autoimmune endocrine diseases but distinguished from APS-1 with its unique triad of hypoparathyroidism, mucocutaneous candidiasis and Addison’s disease and identified mutation of the AIRE gene.
There has been a marked increase in knowledge concerning genetic determinants of disorders such as Type 1A diabetes given whole genome screens analyzing thousands of patients and controls134. The predominant locus influencing Addison’s disease with or without other disorders is the MHC135. The majority of the genes influencing susceptibility outside of the major histocompatibility complex have extremely small odds ratios (often less than 1.2) and thus their analysis in relatively uncommon disorders such as APS-2 is problematic. The major polymorphism (PTPN22 or Lymphocyte Specific Phosphatase R620W) one of the strongest type 1A diabetes non-MHC genes136 is associated with Addison’s disease137-140, but the remainder of the other multiple loci have not been studied for Addison’s disease in detail/ have conflicting findings with limited power141-144. With large genome-wide studies of common disorders such as Graves’ disease and Hashimoto’s thyroiditis multiple immune related genes (e.g. CTLA4, PTPN22, FCRL3) clearly influence disease with relatively small odds ratios145, 146 with evidence of MHC heterogeneity between Graves’ disease and Hashimoto’s thyroiditis145, 147. Loci associated with Addison’s disease outside of the MHC include NALP1 (PR215, minor allele coding SNPrs I2I50220)148 CTLA4-Ala17 (OR = 2.4), Programmed Death Ligand PDLI (OR=1.3)149 and CYP27 (vitamin D I) 150 and vitamin D receptor151.
Genetic abnormalities underlying disease susceptibility for APS-2 and consist primarily of alleles of genes within the major histocompatibility complex. Initial studies associated APS-2 with the class I HLA allele B8 18. HLA-B8 is in linkage dysequilibrium with HLA-DR3, which is in turn is in strong linkage dysequilibrium with DQ2 (DQA1*0501, DQB1*0201). The primary association of APS-2, similar to many autoimmune disorders appears to be with class II HLA alleles (immune response genes) and in particular with DQ2 and DQ8. Thus APS-2 is strongly associated with human leukocyte (HLA) haplotypes with DR3/DQ2 [DQ2:DQA1*0501, DQB1*0201) and DR4/DQ8 (DQ8:DQA1*0301, DQB1*0302) and with DRB1*0404152-154 particularly in the rare multiplex Addison’s disease families) 155-158. A recent study from our group indicates that the association with HLA-B8 is not simply due to its linkage dysequilibrium with DR3-DQ2. In multiplex Addison’s disease families, 95% of DR3 haplotypes have HLA-B8 compared to approximately 50% of control U.S. DR3 haplotypes. The DR3-DQ2-B8 (3.8) of Addison’s disease differs from the usual 3.8.1 (A1) extended haplotype in terms of less often being conserved to A1. This suggests that either B8 itself increases risk or other alleles between DQ and HLA B or B8 itself increases risks and loci between HLA-B and HLA-A decrease Addison’s risk of the 3.8.1 conserved extended haplotype.
Many of the diseases of the type 2 syndrome are associated with HLA antigens HLA-DR3 or HLA-DR4 152, 156, 159 (Table 8.4) Primary adrenal insufficiency in the type 2, but not the type 1 syndrome, is strongly associated with both HLA-DR3 and HLA-DR4152. The association of HLA markers with disease can correlate with inheritance of a common HLA-haplotype within families, but haplotypes with DR3 are often introduced into the family by more than one relative152. Other HLA-B8 and DR3 associated illnesses include selective IgA deficiency160-162 juvenile dermatomyositis, and dermatitis herpetiformis101, alopecia, scleroderma163, autoimmune thrombocytopenia purpura164, hypophysitis108, 109 metaphyseal osteopenia165, and serositis102. A recent report implicates HLA-C as having a greater association with Hashimoto’s thyroiditis than class II HLA alleles, though multiple MHC alleles contribute166.
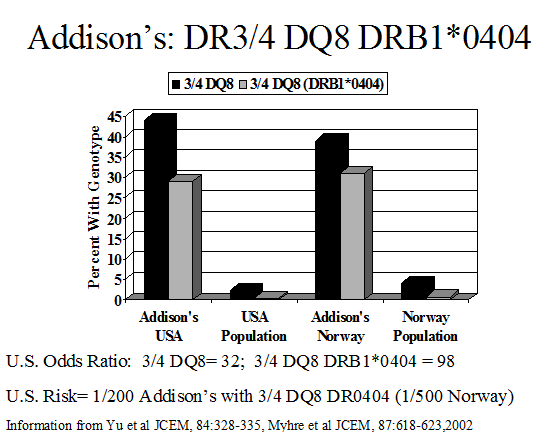
Several diseases that manifest in patients with polyendocrine autoimmune syndromes are not associated with HLA-B8 or HLA-DR3 in population studies. These disorders include vitiligo167, pernicious anemia61, and autoimmune goitrous thyroiditis168-170. Addison’s disease patients also frequently have DQ8, DQB1*0602, and DR5 associated haplotypes(different from patients with type 1 diabetes)135, 171. In our studies, approximately 30% of patients with Addison’s disease are DR3/4, DQ2/DQ8 heterozygous compared to 2.4% of the general U.S. population. This occurs in the U.S. for patients with or without type 1 diabetes. Between 70 to 80% of DR4 alleles in patients with Addison’s disease have DRB1 allele 0404. This is similar to a national study of Addison’s disease patients in Norway153. Such genotypic associations are likely to vary by country depending on the frequency of specific HLA DR and DQ haplotypes. HLA class I alleles also influence risk171
Another allele of an MHC gene that is associated with Addison’s disease is the “5.1” allele of the atypical class I HLA molecule MIC-A172, 173. The MIC-A5.1 allele has a very strong association with Addison’s disease that is not accounted for by linkage dysequilibrium with DR3 or DR4 172, 174, but with extended haplotypes and association of MICA alleles with HLA-B alleles (5.1 with B8 and MICA5 with HLA B15 may not be a causative gene154. The MICA5.1 association is complicated by the protective association of HLA-B15 for Addison’s disease171 and the tight linkage disequilibrium between MIC-A and HLA B alleles. The MHC class II transactivator gene on chromosome 16 influences expression of class II molecules. A single report indicates association of a polymorphism of this gene with a G allele present in 67% of patients with Addison’s disease (n=128) versus 49% of controls154.
ENVIRONMENTAL FACTORS
Initiating factors for the type 2 syndrome and its component illnesses are not established except for celiac disease (wheat protein gliadin) 175, the insulin autoimmune syndrome (e.g. methimizole), myasthenia gravis (rarely penicillamine176), type 1A diabetes (rarely congenital rubella), Graves’ disease (rarely anti-CD52 monoclonal treating patients with multiple sclerosis177) and hypothyroidism (interferon therapy associated with thyroid autoimmunity and diabetes)178-182. A patient treated with interferon alpha developed autoimmune thyroid disease, Addison’s disease and premature ovarian failure that resolved with termination of interferon alpha therapy183.Patients with celiac disease, which is characterized by atrophy of intestinal villi associated with lymphocytic infiltration, have autoantibodies reacting with transglutaminase (the endomysial antigen)129 and with less specificity and sensitivity with the wheat protein gliadin. In contrast to the lack of specificity with gliadin antibody assays, assays of antibodies to deamidated gliadin appear highly specific, and respond to removal of gliadin from the diet more rapidly than transglutaminase autoantibodies184. Removal of gliadin from the diet restores intestinal villi to normal185. There is a well developed hypothesis in terms of the pathogenesis of celiac disease with the deamidation of gliadin by transglutaminase leading to increased binding of the deamidated peptide to HLA DQ2 and DQ8 class II molecules and activation of the disease by presentation of the modified peptides to T lymphocytes186.
Controversial data suggest that ingestion of the milk protein bovine albumin in the first few months of life may be associated with type 1 diabetes187 while other investigators implicate casein, and studies from Denver and Germany implicate early (<3 months) ingestion of wheat. A recent study of omega-3 and omega-6 fatty acid ingestion188 analyzing both dietary history and levels of fatty acids in prospectively obtained red cells associates increased ingestion with decreased risk of developing islet autoimmunity188. These dietary factors appear to increase/decrease risk of islet autoimmunity less than 2-3 fold. A number of drugs are associated with induction of autoimmunity including interferon-a (thyroiditis)189. Remarkably 190, 191 1/3 of multiple sclerosis patients treated with an anti-CD52 monoclonal antibody developed Graves’ disease177. Apparently non-multiple sclerosis patients treated with the same monoclonal do not develop Graves’ disease.
AUTOANTIBODIES
Families with the type 2 polyendocrine syndrome should be evaluated over time to detect the presence of organ-specific antibodies indicating the possibility of a future endocrine malfunction (Table 8.7). All such relatives should be advised of the early symptoms and signs of the principal component diseases. Even though signs and symptoms of disease may be absent, patients with multiple disorders should be screened every few years with measurement of anti-islet antibodies, 21-hydroxylase autoantibodies and transglutaminase autoantibodies, a sensitive thyrotropin assay, and measurement of serum B12 levels192.
Annual ACTH is indicated if 21-hydroxylase autoantibodies are detected152, 194 and then cosyntropin (adrenocorticotropin)-stimulated cortisol determination (cosyntropin test if ACTH is increasing or if symptoms/signs of Addison’s disease are present193). Assays of anti-islet cell antibodies195, 196 anti-thyroid and anti-adrenal antibodies(21-hydroxylase)86, 197-200 and anti-ovarian antibodies104, 201 help identify subjects at increased disease risk. Screening of patients with Type I diabetes for 21-hydroxylase autoantibodies indicates that 1.5% are positive. Approximately 20 percent of APS-2 patients expressing 21-hydroxylase autoantibodies progressed to overt Addison’s disease with long-term follow-up(up to approximately 20 years).193, 202
More than 20 years may elapse between the onset on one endocrinopathy and the diagnosis of the next. As many as 40-50% of subjects with Addison’s disease will develop an associated endocrinopathy. A distinction must be made for subjects with isolated thyroid disease (relatively frequent in the general population) who have no family history of polyglandular syndrome type 2. Such individuals have a relatively low probability of developing additional autoimmune disorders in comparison with individuals with rare autoimmune disorders such as Addison’s disease or myasthenia gravis.
Rarely, hypoparathyroidism, a specific endocrine disturbance present in the type 1 syndrome, is identified in a patient with type 2 syndrome. Hypoparathyroidism in such type 2 polyendocrine autoimmune patients may result from a “suppressive” autoantibody203, 204 rather than the assumed parathyroid destruction as in the type 1 syndrome such as activating antibodies to the calcium receptor 24. Activating antibodies to the calcium receptor appear causative in a subset of patients with hypoparathyroidism24, 205-207 and antibodies to Nalp5 are associated in APS-1 patients25. In a patient with the type 2 syndrome, celiac disease is a more frequent cause of hypocalcemia than hypoparathyroidism.
Several autoantibodies are both disease specific (e.g., anti-acetylcholine receptor antibodies in myasthenia gravis208 and anti-TSH receptor antibodies in Graves’ disease209) and causal. “Causal” autoantibodies are associated with transplacental disease transmission. Other autoantibodies (e.g., antithyroid autoantibodies including anti-thyroid peroxidase, formerly termed anti-microsomal, and anti-thyroglobulin) are as frequent among patients and relatives as to be of little predictive value. For example, a relative with anti-thyroid peroxidase autoantibodies has a low risk of hypothyroidism unless evidence of abnormal thyroid function is also present (e.g., elevated TSH). In a similar manner, many individuals may have antibodies to parietal cells, H+/K+ adenosine triphosphatase210 of the stomach211, 212 and intrinsic factor, but the autoantibodies may not correlate well with abnormal gastric acid secretion or development of pernicious anemia61.
In the APS-2 syndrome, many ICA-positive individuals do not progress to diabetes, and diabetes risk is much lower than for ICA-positive first-degree relatives of patients with type 1 diabetes195, 196. These non-progressing ICA-positive polyendocrine patients usually express what has been termed “selective” or restricted ICA195. Such ICA reacts only with islet B cells (insulin producing), not A cells (glucagon producing) within rat islets and fail to react with mouse islets. They represent unusual high titer autoantibodies reacting with glutamic acid decarboxylase (GAD), which is not expressed at detectable levels in mouse islets. This unusual form of ICA confers a lower risk of type 1 diabetes as compared with nonrestricted ICA (reacts with multiple islet molecules) for both polyendocrine patients and relatives of patients with type 1 diabetes. If multiple anti-islet autoantibodies (of GAD, insulin and IA-2) are present, there is a high risk of diabetes.
|
Table 8.7. Type 1A Diabetes and Polyendocrine Autoimmunity
|
|
· As many as 18% of APS-1 patients become diabetic as do approximately 15% of patients with Addison’s disease (non-APS-I) · “Restricted” or GAD-ICA is common among APS 2 patients with lower risk of progression to diabetes unless IA-2 autoantibodies are present · APS-1 and 2: DQA1*0102/DQB1*0602 Protection from Diabetes · APS-2: Later mean age of diabetes onset than typical type 1A patients · APS-I: Anti-GAD autoantibodies react with GAD on Western blots and inhibit enzymatic activity 213
|
Other autoantibodies associated with the type 2 syndrome include anti-melanocytic, anti-adrenal 60 and anti-gonadal autoantibodies198. Anti-adrenal cortical antibodies have been used to predict adrenal insufficiency in the type 1 syndrome (in particular 21-hydroxylase). In particular amongst patients with 21-hydoxylase autoantibodies, follow up with annual ACTH levels allows early diagnosis193. Addison’s disease is associated with premature ovarian failure and antibodies to steroid cells predictive37. It is noteworthy that many of the polyendocrine autoantibodies react with intracellular enzymes, including thyroid peroxidase (Hashimoto’s thyroiditis), glutamic acid decarboxylase (type 1 diabetes and stiff-man syndrome), 21 hydroxylase (Addison’s disease), and cytochrome P450 cholesterol side chain cleavage enzyme117 (Addison’s disease). In addition, antibodies to hormones can be present, including anti-insulin, anti-thyroxine, and anti-intrinsic factor antibodies (pernicious anemia). It is now relatively easy to develop highly specific and sensitive assays for autoantibodies reacting with in vitro transcription and translation of cDNAs of the relevant protein. The specificity of ELISA format assays can be enhanced with competition with fluid phase molecules and detection with fluorescence assays214. Dr. Hutton and colleagues utilized identification of tissue specificity followed by development of fluid phase radioassay to define the fourth major islet autoantigen, namely the beta cell Zinc transporter(ZnT8)215.
Antibodies to specific receptors are characteristic of given disorders (anti-acetylcholine receptor antibodies of myasthenia gravis, anti-TSH receptor antibodies of Graves’ disease or hypothyroidism216, and oocyte sperm receptor autoantibodies associated with oophoritis). The large variety of target molecules, (e.g., type 1 diabetes), presence of high affinity IgG autoantibodies, and the sequential appearance over months or years of specific antibodies or disorders suggest that the production of most autoantibodies is secondary to tissue destruction and are antigen “driven”217.
PATHOGENESIS
A central question is what links all the different disorders of the APS-2 syndrome? Why do some individuals have a single autoimmune disorder while others have multiple diseases?
One hypothesis is that different tissues share the same autoantigen and thus when autoimmunity is directed at one organ it will also affect other organs. This is highly unlikely given the number of different molecules targeted specifically for many autoimmune disorders and the wide discordance in time relative to the appearance of for instance specific autoantibodies and disease. Another hypothesis is that different organs may share immunologically related molecules (mimics) and such mimics may be as simple as short peptides recognized by T lymphocytes. That is also a possibility (see below), but would not explain the wide time differences of disease appearance and spectrum of different illnesses. We believe the most likely link between the diverse diseases is genetic propensity to fail to maintain tolerance to multiple self-molecules, and in particular specific self-peptides. Environmental factors and additional genetic determinants (e.g. specific HLA alleles) then determine the timing of loss of tolerance and the probability that a specific organ will be targeted. For instance, the highest risk (for Type 1 diabetes) HLA genotype DR3-DQ2; DR4-DQ8 is associated with a young age of diabetes onset. Failure to maintain tolerance can be a result of deficient T regulation or enhanced T cell activation. An additional hypothesis is that HLA alleles associated with autoimmunity might be inherently contributing to generalized autoreactivity. We find that hypothesis unattractive in that specific HLA haplotypes can be protective for one autoimmune disorder and promote another. For example DR2/DQB1*0602 haplotypes are high risk for multiple sclerosis but provide dominant protection for type 1A diabetes.
Both autoreactive T cells and autoantibodies can be pathogenic, depending on the specific disease. In Graves’ disease, anti-thyrotropin (TSH) autoantibodies lead to thyroid hyperfunction218 and anti-insulin receptor autoantibodies can result in either hypoglycemia or insulin resistance with hyperglycemia15, 219. Type 1A diabetes is a T cell mediated disorder and an interesting case report describes a child developing diabetes with a mutation eliminating B-lymphocytes and thus autoantibodies (59). Nevertheless, the anti-B cell monoclonal Rituximab (anti-CD20) slowed progression of C-peptide loss in new onset diabetic patients. 220
T cell autoimmunity has been much more difficult to study and correlate with disease compared to autoantibodies. Recent advances in T cell immunobiology, studies of animal models, and transfer of autoreactive T lymphocytes or affected human organs into immunodeficient mice should lead to progress in understanding T cell autoimmunity (See chapter on T lymphocytes). For instance, as expected the 21 hydroxylase molecule is a T cell target in Addison’s disease221, with multiple peptides of 21-hydroxylase presented by HLA-B8 to patient T lymphocytes.
For decades, experimental animal models of organ-specific autoimmunity have been studied. These were dependent upon the injection of putative autoantigens into animals in the presence of adjuvants that enhance inflammation107. Thus, thyroiditis can readily be induced in selective strains of mice following injection of thyroglobulin or thyroid peroxidase in Freund’s adjuvant. Anti-insulin autoantibodies can be induced in normal Balb/c mice following the administration of insulin peptide B:9-23, and these autoantibodies react with intact insulin and are not absorbed by the immunizing peptide222. In Balb/c mice expressing an activating molecule in islets (B7.1) immunization with the B:9-23 peptide leads to diabetes. T cell clones reacting with these molecules, or other selected peptides, are generated, and such clones when transferred into naive animals induce disease. Of note, several forms of immunization with such autoreactive clones can be used to make animals refractory to disease induction223. These studies provide clear evidence that autoreactive T cells are present in normal animals and they can be rapidly activated, given “appropriate” stimulation.
In addition, studies by Tung and associates and Wucherpfennig and colleagues suggests one mechanism whereby properties of T cell recognition may lead to multiple autoimmune disorders107, 224. In studying experimental autoimmune oophoritis, Tang and colleagues identified a peptide of the oocyte sperm receptor (ZP3) that upon injection in adjuvant induced disease. They then identified which of nine amino acids of this peptide were essential to activate autoimmune T cell clones or induce disease. T cells recognize only short peptides presented in the groove of class I or class II major histocompatibility complex (MHC) molecules on antigen-presenting cells. As few as three properly spaced amino acids (of nine) interacting with a T cell receptor can be sufficient to trigger T cell responses. Noting that a peptide of the acetylcholine receptor had the appropriate T cell binding motif associated with experimental oophoritis, they demonstrated that this peptide stimulated an oophoritis-derived T cell clone in vitro and when administered in vivo induced oophoritis. The requirement for sharing of as few as three of nine amino acids of a linear sequence for activation of autoreactive T cells provides a mechanism whereby inflammation directed at one organ may spread to additional tissues by T cell cross reactions to distinct peptides of different tissues. Such a model may also help explain disease associations. For example, Graves’ thyroid disease is frequently complicated by autoimmunity directed at extraocular muscles leading to Graves’ ophthalmopathy225. If such a mechanism for the “spreading” of autoimmunity is operative, it implies that mechanisms to suppress autoreactivity are particularly important. The possibilities that “molecular mimicry” may induce autoimmunity are greatly increased if short minimally homologous sequences are sufficient to stimulate cross-reactive T cell clones.
Very important animal models of polyendocrine autoimmunity indicate that loss of regulatory T lymphocytes can result in widespread autoimmunity. These models utilize either neonatal thymectomy or a combination of radiation and immunosuppressive drugs and transfer of T cell subsets to immunodeficient mice to induce autoimmunity 226-232. The general concept is that within the thymus, early in development, a subset of essential regulatory T lymphocytes develops and seeds the periphery. Interference with this normal mechanism results in loss of tolerance to multiple molecules, and thus multiple organs are the target of autoimmunity. This is a rapidly developing field. The IPEX syndrome (see below) with its fatal neonatal autoimmunity and loss of a key regulatory molecule (Foxp3) illustrates how this concept can apply to human autoimmune disorders233.
Kriegel and co-workers reported that patients with APS-2 have a defect in terms of lymphocyte response to CD4+CD25+ T cell suppression 252. Interruption of normal T cell development can result in multiple autoimmune disorders. The BB rat develops type 1 diabetes and thyroiditis in association with a severe T cell immunodeficiency253. Neonatal thymectomy induces autoimmunity apparently by removing regulatory T cells254, 255. A series of different regulatory T cells (e.g. CD4+CD25+, NK T cells) are now the subject of intense investigation 256. Of note the Foxp3 molecule is essential for regulatory T lymphocytes and when it is mutated (IPEX syndrome-see below) neonatal autoimmune diabetes results257, 258.
Autoimmune disorders appear to share a number of “non-specific” abnormalities of T cell function or enumeration including increased numbers of cells expressing class II molecules (“Ia” positive T cells)259, IL2 receptors, depressed autologous mixed lymphocyte responses260, and lack of NK T cells261. Studies of NK T cells in man are controversial with tetramer analysis not confirming decreased numbers in patients with type 1A diabetes, but rather stable wide variation in the percentage of such cells between even normal individuals262. The above abnormalities appear not to be disease specific and may relate to fundamental abnormalities predisposing to autoimmunity or reflect disease activity.
IDENTIFICATION OF CASES
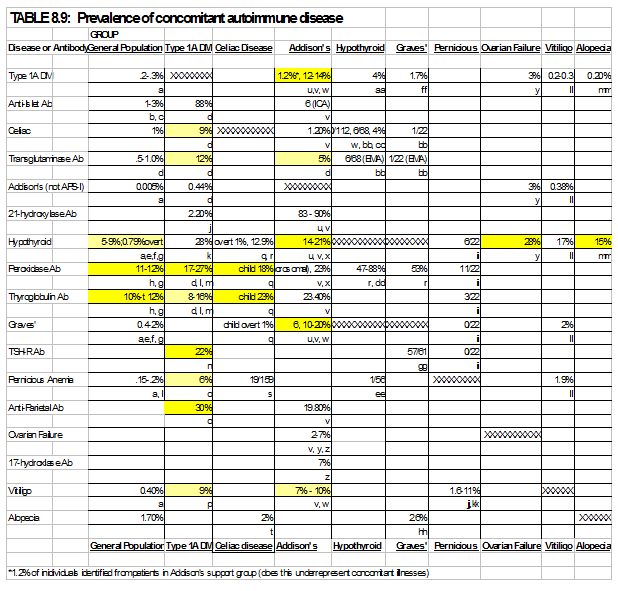
Individuals with a single autoimmune disease are at increased risk for the development of a second disease compared to the general population. Table 8.9 shows the prevalence of autoimmune endocrine disease in the general population and the co-incidence of a second autoimmune disease given that a first exists. In addition, individuals with APS-2 syndrome will often develop autoimmunity sequentially over the time course of many years. An individual often will not have polyglandular failure at the onset of clinical symptoms of the initial autoimmune disease. Therefore, a high clinical suspicion for the development of sequential autoimmune diseases must be maintained 263. Specific screening strategies depend on the presenting autoimmune disease. Significant controversy exists regarding the screening tests that should be employed and the frequency of testing performed. For example, in type 1 diabetes, it is generally accepted that routine screening for thyroid disease with biochemical assays should be performed (e.g. TSH assay), through the frequency of this screening is a source of controversy 264; 265. Even more controversial is the screening for celiac disease in this population. While the elevated risk of celiac disease in the diabetic population has been well established 129, 266-267, many of these individuals are asymptomatic at the time of identification and the long term sequelae of untreated asymptomatic celiac disease in regards to growth, pubertal development, bone mineralization and gastrointestinal malignancy is unclear. To avoid “negative” celiac intestinal biopsies, we refer for biopsy primarily patients with high levels of transglutaminase autoantibodies. Increased levels of transglutaminase autoantibodies are strongly associated with a positive celiac biopsy268. The appropriate level is assay specific. For the fluid phase radioassay we utilize, an index of 0.05 represents the 99th percentile of normal controls while a level of 0.5 is the cutoff we utilize for obtaining biopsy.
Studies of anti-pituitary (anti-hypothalmic)269 autoimmunity have progressed in the past decade270, 271 including identification of IgG4 hypophysitis271, anti-Pit1 combined pituitary deficiencies272 and description of predictive pituitary antibody staining pattern131. Bellastella and coworkers have reported that 99 of 700 of APS patients (primarily patients with thyroid autoimmunity type 1 diabetes mellitus and gastritis) had autoantibodies reacting with isolated pituitary cells (not a diffuse staining pattern)131. Despite normal MRI with 5 years of follow up, 19% with this immunostaining pattern developed one or more pituitary hormone deficiency. Patients with diffuse pituitary antibody staining did not develop hypopituitarism.
Tests may include functional biochemical assays and/or serologic studies to identify organ specific autoantibodies. Once a second autoimmune disease is identified more extensive screening is indicated to identify further disease at an early stage. Screening with autoantibodies associated with diabetes (IA-2, insulin and GAD), thyroid disease (TG and/or TPO), Addison’s disease (21-hydroxylase), celiac disease (transglutaminase) and autoimmune hepatitis (cytochrome P450 enzymes) in addition to biochemical screening with TSH, free T4, FSH, LH, CBC, and electrolytes may uncover occult autoimmune disease.

Therapy
The therapy of the APS-2 syndrome depends upon the specific disease manifestation with a few caveats273. Patients with suspected Addison’s disease and hypothyroidism should be evaluated and treated for adrenal insufficiency prior to replacement of thyroid hormone to avoid Addisonian crisis. There is one fascinating case report of a patient with 21-hydroxylase autoantibodies treated for Graves’ eye disease with a 6 month course of glucocorticoids. In this patient 21-hydroxylase autoantibodies became negative and adrenal function was restored to normal. This remission was reported to have lasted for 100 months at last follow up274.
There are a large number of new potent immunosuppressive and immunomodulatory therapies being used in non-endocrine autoimmune diseases and in various stages of clinical development. Rituximab (anti-CD20 antibody) is one of the more interesting having dramatic effects in multiple sclerosis, transient slowing of loss of c-peptide in patients with new onset diabetes275, 276 and a case report of response of an APS-1 patient with pulmonary disease36 as well as a relatively large clinical experience for the treatment of B-cell lymphomas. In the NOD mouse model of type 1 diabetes anti-CD20 prevents development of diabetes277 and a clinical trial in new onset patients (Trialnet) indicates a single course slows but does not permanently arrest loss of C-peptide. Treatment with rituximab induces long-term B cell depletion, but the antibody does not bind to plasma cells, and often has relatively minor effects on autoantibody levels. It may act by influencing presentation of autoantigens by B-lymphocytes or altering B cell regulation. Studies in Graves’ disease suggest a potential role, but also potential complications 278-280. Trials of modified anti-CD3 monoclonal antibodies in new onset type 1 diabetes are very advanced with the potential that such antibodies induce regulatory T lymphocytes as well as acutely and transiently depleting T lymphocytes 281-286. A long-term goal is the development of antigen specific therapy for each of the major autoimmune disorders. In experimental animals regulatory T lymphocytes targeting for instance islet specific molecules can effectively block development of disease287, 288.
IPEX (Immune Dysfunction Polyendocrinopathy X-linked)
The IPEX syndrome presents in neonates with fatal autoimmunity and this very rare disorder has multiple different names reflecting endocrinopathy, allergic manifestations, intestinal destruction and immune dysregulation (e.g. XLAAD: X-Linked Autoimmunity Allergic-Dysregulation Syndrome or XPID, MIM number 304790 and 300292). Children with the disorder can die in infancy and many die in the first days of life but there is heterogeneity with some children surviving 12-15 years233. They manifest neonatal type 1 diabetes, but the cause of death probably relates to massive intestinal involvement and malabsorption. Eighty percent of patients with the IPEX syndrome develop type 1 diabetes. This suggests that in the absence of regulatory T lymphocytes most humans will target and destroy beta cells.289
The disease results from mutations that inactivate the Foxp3 transcription factor and the same gene is also mutated in a mouse model (the Scurfy mouse)6. The pathway this gene controls in T lymphocytes is now identified as central to basic immunology. In particular the gene controls the regulatory function of CD4+CD25+ regulatory T lymphocytes. 227, 290 From this discovery it is now apparent why bone marrow transplantation of normal lymphocytes is able to cure the mouse disease, namely the replacement of regulatory lymphocytes is able to control autoimmune reactivity of effector lymphocytes of the Scurfy mouse recipient, despite their lacking the Foxp3 gene.
In that the mouse model is cured with bone marrow transplantation, such therapy has recently been tested in children with the disorder. A child became chimeric after bone marrow transplantation and a remarkable two-year remission was induced(142), followed by an unusual hematological disease that resulted in death. Partial lymphoid chimerism following bone marrow transplantation can induce remission 291. There are now multiple reports of bone marrow transplantation 291-293 and a report of therapy with Sirolimus294.
Anti-Insulin Receptor Antibodies
The presence of anti-insulin receptor autoantibodies is characterized by marked insulin resistance, but paradoxically, patients can also have severe hypoglycemia9. Approximately one third of the subjects have other autoimmune disorders. Characteristically, associated autoimmune diseases are non-organ specific295-297. A recent intramural NIH study indicates that a regimen of Rituximab, cyclophosphamide and pulse steroids can induce remission of the disease298.
Thymic Tumors
Thymomas and thymic hyperplasia are associated with a series of autoimmune diseases10-12, 299-301. The most common autoimmune diseases are myasthenia gravis and red cell aplasia302, 303. Graves’ disease, type 1 diabetes, and Addison’s disease may also be associated with thymic tumors. Patients with myasthenia gravis and alopecia totalis are reported to have thymoma 304. Unique anti-acetylcholine receptor autoantibodies may be present with thymoma301 and disease may be initiated by transcription of molecules within the tumor related to acetylcholine receptors305. There is a 1997 report of treatment of a patient with thymoma and pure red cell aplasia with octreotide and prednisone299. Many thymomas lack AIRE expression within the thymoma a potential factor in the development of autoimmunity306, 307. Of note, the one other disease with “frequent” development of anti-cytokine (e.g. anti-inferon and ILI7 (autoantibodies besides AIRE mutations are thymoma associated308). A patient described by Anderson and coworkers with thymoma had acquired polyglandular syndrome and AIRE defect13.
POEMS Syndrome
POEMS (Polyneuropathy, Organomegaly, Endocrinopathy, M-protein, Skin changes) patients usually present with a sensory motor polyneuropathy, diabetes mellitus (50%), primary gonadal failure (70%), and a plasma cell dyscrasia with sclerotic bony lesions309 17, 310. Temporary remission may result following radiotherapy directed at the plasmacytoma and peripheral blood stem cell transplantation has been utilized 311. The syndrome is assumed to be secondary to circulating immunoglobulins but patients have excess vascular endothelial growth factor312, 313 as well as elevated IL1-b, IL-6, and TNF-a310. There is a case report of a presumptive patient with POEMS without polyneuropathy314. A small series of patients have been treated with thalidomide with decrease in VEGF315. A number of centers have extensive experience with autologous stem cell transplantation{26986}{26987}.
Insulin Autoimmune Syndrome (Hirata Syndrome)
The insulin autoimmune syndrome, associated with Graves’ disease and methimazole therapy (or other sulfhydryl containing medications) is of particular interest due to a remarkably strong association with a specific HLA haplotype 15. Such patients with elevated titers of anti-insulin autoantibodies frequently present with hypoglycemia. The disease in Japan is essentially confined to DR4-positive individuals with DRB1*0406239. Even a Portuguese patient with the syndrome had DRB1*0406316. In Hirata syndrome the anti-insulin autoantibodies are polyclonal. Some patients have monoclonal anti-insulin autoantibodies that also induce hypoglycemia and for these patients there is not an HLA association316.
Adult combined Pituitary Hormone Deficiency (CPHD) with Anti-Pit1 Autoantibodies
Yamamoto and coworkers recently described three patients with multiple autoimmune disorders and GH, prolactin and TSH deficiency. In that Pit-1 is a transcription factor essential for production of these pituitary hormones they searched for and found specifically in these three patients autoantibodies to pit-1. In addition, one of the patients with the anti-Pit 1 autoantibodies, lacked pituitary cells producing these hormones272.
Other Disorders
Other diseases with polyendocrine manifestations are Kearns-Sayre syndrome317 diabetes and thyroiditis associated with trisomy 21318, DIDMOAD syndrome (diabetes insipidus, diabetes mellitus, optic atrophy, and nerve deafness, also termed Wolfram syndrome)319-321 and congenital rubella240, 322, 323 associated with thyroiditis and/or diabetes.
The Kearns-Sayre syndrome is characterized by onset before age 20, external ophthalmoplegia, pigmentary retinal degeneration, and one or more of ataxia, heart block, or high cerebrospinal fluid protein. A number of these patients have Hashimoto’s thyroiditis with the suggestion that hypothyroidism is associated with encephalopathy 324, hypoparathyroidism325 and diabetes mellitus and adrenal insufficiency326. The disorder is associated with multiple large-scale327 deletions as well as mutations of mitochondrial DNA 328.
Wolfram syndrome (DIDMOAD syndrome) is characterized by optic atrophy and childhood onset diabetes but the diabetes is not of autoimmune etiology. The gene mutated (wolframin on chromosome 4p16) encodes a glycosylated transmembrane protein of unknown function319, 329 that is localized to the endoplasmic reticulum (ER) and may function to limit ER-stress induced cell death 330.
Diabetes develops in a significant number of adolescents and young adults with a history of congenital rubella infection. These patients frequently have thyroiditis, and despite the development of childhood diabetes infrequently express anti-islet autoantibodies 331. Karounos and coworkers have described a rubella protein with homology to an islet protein, suggesting that molecular mimicry may initiate disease332. An alternative hypothesis has been proposed by Rabinowe and coworkers323. They described long-term T cell subset abnormalities in patients following congenital rubella infection.
Conclusions
Polyendocrine autoimmune syndromes have played an important role in understanding autoimmune disorders and in particular type 1A diabetes. The initial evidence that type 1A diabetes was an autoimmune disorder came from its association with spontaneous Addison’s disease, and lack of association with tuberculous Addison’s disease5. The first demonstration of cytoplasmic islet cell autoantibodies occurred in patients with polyendocrine autoimmunity333.
The existence of families of related autoimmune disorders is not only clinically important but also suggests that these diseases are pathogenically related. This relationship probably is a result of two distinct phenomena. The first is inherited abnormalities of immune function, predisposing to the loss of tolerance to a series of self-antigens. The IPEX syndrome with lack of CD4-CD25 regulatory T cells is very instructive with 80% of such children developing type 1 diabetes. Given such a predisposition, “normal” alleles of HLA genes within the major histocompatibility complex may then lead to targeting of specific organs. The second phenomenon that may link these disorders, especially for tightly linked diseases such as Graves’ ophthalmopathy and Graves’ thyroid disease, is creation of pathogenic T or B 334 cells reacting with components of more than one tissue. In that T cell clones can respond to peptides that share no identical amino acids 335, depending upon their three dimensional structure, the potential for such T-cell cross-reactivity must be enormous 334.
Finally, the relationships between these diverse disorders suggest that as disease pathogenesis is elucidated and antigen-specific therapies are developed, the improved understanding of pathogenesis and improvements in therapy will be applicable to many autoimmune diseases.
Reference List
(1) Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy - candidiasis - ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med 1990;322(26):1829-36.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=2348835&query_hl=4&itool=pubmed_docsum
(2) Betterle C, Ghizzoni L, Cassio A, Baronio F, Cervato S, Garelli S, et al. Autoimmune-Polyendocrinopathy-Candidiasis-Ectodermal- Dystrophy (APECED) in Calabria: clinical, immunological and genetic patterns. J Endocrinol Invest 2011 Nov 21.PM:22104652
(3) Meloni A, Willcox N, Meager A, Atzeni M, Wolff AS, Husebye ES, et al. Autoimmune polyendocrine syndrome type 1: an extensive longitudinal study in Sardinian patients. J Clin Endocrinol Metab 2012 Apr;97(4):1114-24.PM:22344197
(4) Schmidt MB. Eine biglandulare Erkrankung (Nebennieren und Schilddruse) bei Morbus Addisonii. Verh Dtsch Ges Pathol 1926;21:212-21
(5) Nerup J. Addison's disease - clinical studies: A report of 108 cases. Acta Endocrinol 1974;76:127-41.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=4609691&query_hl=5
(6) Patel DD. Escape from tolerance in the human X-linked autoimmunity-allergic disregulation syndrome and the Scurfy mouse. J Clin Invest 2001;107(2):155-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11160129&query_hl=7
(7) Husebye ES, Anderson MS. Autoimmune polyendocrine syndromes: clues to type 1 diabetes pathogenesis. Immunity 2010 Apr 23;32(4):479-87.PM:20412758
(8) Flier JS, Kahn CR, Roth J, Bar RS. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science 1975;190:63-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=170678
(9) Flier JS, Bar RS, Muggeo M, Kahn CR, Roth J, Gorden P. The evolving clinical course of patients with insulin receptor autoantibodies: Spontaneous remission or receptor proliferation with hypoglycemia. J Clin Endocrinol Metab 1978;47:985-95.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=263346
(10) Souadjian JV, Enriquez P, Silverstein MN, Pepin J-M. The spectrum of diseases associated with thymoma. Arch Intern Med 1974;134:374-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=4602050
(11) Combs RM. Malignant thymoma, hyperthyroidism and immune disorder. South Med J 1968;61:337-41.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=5644070
(12) Maggi G, Casadio C, Cavallo A, Cianci R, Molinatti M, Ruffini E. Thymoma: results of 241 operated cases. Ann Thorac Surg 1991;51:152-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1985561
(13) Cheng MH, Fan U, Grewal N, Barnes M, Mehta A, Taylor S, et al. Acquired autoimmune polyglandular syndrome, thymoma, and an AIRE defect. N Engl J Med 2010 Feb 25;362(8):764-6.PM:20181983
(14) Hirata Y, Ishizu H, Ouchi N, Motumura S, Abe M, Hara Y, et al. Insulin autoimmunity in a case with spontaneous hypoglycaemia. Japan J Diabetes 1970;13:312-9
(15) Uchigata Y, Hirata Y. Insulin Autoimmune Syndrome (IAS, Hirata Disease). In: Eisenbarth G, editor. Molecular Mechanisms of Endocrine and Organ Specific Autoimmunity.Austin, Texas: R.G.Landes; 1999. p. 133-48.
(16) Imawari M, Akatsuka N, Ishibashi M, Beppu H, Suzuki H, Yoshitoshi Y. Syndrome of plasma cell dyscrasia, polyneuropathy, and endocrine disturbances. Ann Intern Med 1974;81:490-3.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=4416017
(17) Miralles GD, O'Fallon JR, Talley NJ. Plasma-cell dyscrasia with polyneuropathy: the spectrum of POEMS syndrome. N Engl J Med 1992;327(27):1919-23.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1333569
(18) Eisenbarth GS, Wilson P, Ward F, Lebovitz HE. HLA type and occurrence of disease in familial polyglandular failure. N Engl J Med 1978;298(2):92-4.http://www.ncbi.nlm.nih.gov/pubmed/619238?ordinalpos=10&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(19) Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab 2006 Aug;91(8):2843-50.http://www.ncbi.nlm.nih.gov/pubmed/16684821?ordinalpos=4&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(20) Betterle C. Parathyroid and autoimmunity. Ann Endocrinol (Paris) 2006 Apr;67(2):147-54.http://www.ncbi.nlm.nih.gov/pubmed/16639366?ordinalpos=7&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(21) Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med 2004 May 13;350(20):2068-79.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=15141045&query_hl=3&itool=pubmed_docsum
(22) Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol 2007 Aug;7(8):645-50.http://www.ncbi.nlm.nih.gov/pubmed/17641664?dopt=Citation
(23) Tomar N, Kaushal E, Das M, Gupta N, Betterle C, Goswami R. Prevalence and significance of NALP5 autoantibodies in patients with idiopathic hypoparathyroidism. J Clin Endocrinol Metab 2012 Apr;97(4):1219-26.PM:22278434
(24) Gavalas NG, Kemp EH, Krohn KJ, Brown EM, Watson PF, Weetman AP. The calcium-sensing receptor is a target of autoantibodies in patients with autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab 2007 Jun;92(6):2107-14
(25) Alimohammadi M, Bjorklund P, Hallgren A, Pontynen N, Szinnai G, Shikama N, et al. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med 2008 Mar 6;358(10):1018-28.http://www.ncbi.nlm.nih.gov/pubmed/18322283?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(26) Ahlgren KM, Moretti S, Lundgren BA, Karlsson I, Ahlin E, Norling A, et al. Increased IL-17A secretion in response to Candida albicans in autoimmune polyendocrine syndrome type 1 and its animal model. Eur J Immunol 2011 Jan;41(1):235-45.PM:21182094
(27) Cludts I, Meager A, Thorpe R, Wadhwa M. Detection of neutralizing interleukin-17 antibodies in autoimmune polyendocrinopathy syndrome-1 (APS-1) patients using a novel non-cell based electrochemiluminescence assay. Cytokine 2010 May;50(2):129-37.PM:20116277
(28) Kisand K, Lilic D, Casanova JL, Peterson P, Meager A, Willcox N. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur J Immunol 2011 Jun;41(6):1517-27.PM:21574164
(29) Bialkowska J, Zygmunt A, Lewinski A, Stankiewicz W, Knopik-Dabrowicz A, Szubert W, et al. Hepatitis and the polyglandular autoimmune syndrome, type 1. Arch Med Sci 2011 Jun;7(3):536-9.PM:22312376
(30) Ekwall O, Sjoberg K, Mirakian R, Rorsman F, Kampe O. Tryptophan hydroxylase autoantibodies and intestinal disease in autoimmune polyendocrine syndrome type 1 [letter]. Lancet 1999 Aug 14;354(9178):568.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10470707
(31) Posovszky C, Lahr G, von SJ, Buderus S, Findeisen A, Schroder C, et al. Loss of enteroendocrine cells in autoimmune-polyendocrine-candidiasis-ectodermal-dystrophy (APECED) syndrome with gastrointestinal dysfunction. J Clin Endocrinol Metab 2012 Feb;97(2):E292-E300.PM:22162465
(32) Oliva-Hemker M, Berkenblit GV, Anhalt GJ, Yardley JH. Pernicious anemia and widespread absence of gastrointestinal endocrine cells in a patient with autoimmune polyglandular syndrome type I and malabsorption. J Clin Endocrinol Metab 2006 Aug;91(8):2833-8
(33) Garty BZ, Kauli R. Alopecia universalis in autoimmune polyglandular syndrome type I. West J Med 1990;152:76-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2309482
(34) Ahonen P, Miettinen A, Perheentupa J. Adrenal and steroidal cell antibodies in patients with autoimmune polyglandular disease type I and risk of adrenocortical and ovarian failure. J Clin Endocrinol Metab 1987;64(3):494-500.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3818889
(35) De Luca F, Valenzise M, Alaggio R, Arrigo T, Crisafulli G, Salzano G, et al. Sicilian family with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) and lethal lung disease in one of the affected brothers. Eur J Pediatr 2008 Feb 15;.http://www.ncbi.nlm.nih.gov/pubmed/18274776?ordinalpos=29&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(36) Popler J, Alimohammadi M, Kampe O, Dalin F, Dishop MK, Barker JM, et al. Autoimmune polyendocrine syndrome type 1: Utility of KCNRG autoantibodies as a marker of active pulmonary disease and successful treatment with rituximab. Pediatr Pulmonol 2012 Jan;47(1):84-7.PM:21901851
(37) Reato G, Morlin L, Chen S, Furmaniak J, Rees SB, Masiero S, et al. Premature Ovarian Failure in Patients with Autoimmune Addison's Disease: Clinical, Genetic, and Immunological Evaluation. J Clin Endocrinol Metab 2011 Jun 15.PM:21677034
(38) Cakir ED, Ozdemir O, Eren E, Saglam H, Okan M, Tarim OF. Resolution of autoimmune oophoritis after thymectomy in a myasthenia gravis patient. J Clin Res Pediatr Endocrinol 2011;3(4):212-5.PM:22155465
(39) Alkaabi JM, Chik CL, Lewanczuk RZ. Pericarditis with cardiac tamponade and addisonian crisis as the presenting features of autoimmune polyglandular syndrome type II: a case series. Endocr Pract 2008 May;14(4):474-8
(40) Berger JR, Weaver A, Greenlee J, Wahlen GE. Neurologic consequences of autoimmune polyglandular syndrome type 1. Neurology 2008 Jun 3;70(23):2248-51
(41) Solimena M, De Camilli P. From Th1 to Th2: diabetes immunotherapy shifts gears. Nat Med 1996;2:1311-2
(42) Pollak U, Bar-Sever Z, Hoffer V, Marcus N, Scheuerman O, Garty BZ. Asplenia and functional hyposplenism in autoimmune polyglandular syndrome type 1. Eur J Pediatr 2008 May 22;.http://www.ncbi.nlm.nih.gov/pubmed/18496713?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(43) Rosa DD, Pasqualotto AC, Denning DW. Chronic mucocutaneous candidiasis and oesophageal cancer. Med Mycol 2008 Feb;46(1):85-91
(44) Valenzise M, Meloni A, Betterle C, Giometto B, Autunno M, Mazzeo A, et al. Chronic inflammatory demyelinating polyneuropathy as a possible novel component of autoimmune poly-endocrine-candidiasis-ectodermal dystrophy. Eur J Pediatr 2008 May 7;.
(45) Mandel M, Etzioni A, Theodor R, Passwell JH. Pure red cell hypoplasia associated with polyglandular autoimmune syndrome type I. Isr J Med Sci 1989;25:138-41.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2496049
(46) Hara T, Mizuno Y, Nagata M, Okabe Y, Taniguchi S, Harada M, et al. Human gamma delta T-cell receptor-positive cell-mediated inhibition of erythropoiesis in vitro in a patient with type I autoimmune polyglandular syndrome and pure red blood cell aplasia. Blood 1990;75:941-50.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2105751
(47) Friedman TC, Thomas PM, Fleisher TA, Feuillan P, Parker RI, Cassorla F, et al. Frequent occurrence of asplenism and cholelithiasis in patients with autoimmune polyglandular disease type I. Am J Med 1991;91:625-30.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1750432
(48) Hogenauer C, Meyer RL, Netto GJ, Bell D, Little KH, Ferries L, et al. Malabsorption due to cholecystokinin deficiency in a patient with autoimmune polyglandular syndrome type I. N Engl J Med 2001 Jan 25;344(4):270-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11172154
(49) Gianani R, Eisenbarth GS. Autoimmunity to gastrointestinal endocrine cells in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab 2003 Apr;88(4):1442-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12679419
(50) Posovszky C, Lahr G, von SJ, Buderus S, Findeisen A, Schroder C, et al. Loss of enteroendocrine cells in autoimmune-polyendocrine-candidiasis-ectodermal-dystrophy (APECED) syndrome with gastrointestinal dysfunction. J Clin Endocrinol Metab 2012 Feb;97(2):E292-E300.PM:22162465
(51) Skoldberg F, Portela-Gomes GM, Grimelius L, Nilsson G, Perheentupa J, Betterle C, et al. Histidine decarboxylase, a pyridoxal phosphate-dependent enzyme, is an autoantigen of gastric enterochromaffin-like cells. J Clin Endocrinol Metab 2003 Apr;88(4):1445-52.http://www.ncbi.nlm.nih.gov/pubmed/12679420?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(52) Korniszewski L, Kurzyna M, Stolarski B, Torbicki A, Smerdel A, Ploski R. Fatal primary pulmonary hypertension in a 30-yr-old female with APECED syndrome. Eur Respir J 2003 Oct;22(4):709-11.http://www.ncbi.nlm.nih.gov/pubmed/14582926?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(53) Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 2010 Feb 15;207(2):291-7.PM:20123958
(54) Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 2010 Feb 15;207(2):299-308.PM:20123959
(55) Meager A, Visvalingam K, Peterson P, Moll K, Murumagi A, Krohn K, et al. Anti-Interferon Autoantibodies in Autoimmune Polyendocrinopathy Syndrome Type 1. PLoS Med 2006 Jun 13;3(7):e289.http://www.ncbi.nlm.nih.gov/pubmed/16784312?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(56) Kumar PG, Laloraya M, She JX. Population genetics and functions of the autoimmune regulator (AIRE). Endocrinol Metab Clin North Am 2002 Jun;31(2):321-38, vi.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12092453
(57) Su MA, Giang K, Zumer K, Jiang H, Oven I, Rinn JL, et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest 2008 May;118(5):1712-26.http://www.ncbi.nlm.nih.gov/pubmed/18414681?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(58) Aaltonen J, Björses P, Sandkuijl L, Perheentupa J, Peltonen L. An autosomal locus causing autoimmune disease: autoimmune polyglandular disease type I assigned to chromosome 21. Nat Genet 1994 Sep;8(1):83-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7987397
(59) Aaltonen J, Björses P, Perheentupa J, Horelli-Kuitunen N, Palotie A, Peltonen L, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 1997;17(4):399-403
(60) Myhre AG, Halonen M, Eskelin P, Ekwall O, Hedstrand H, Rorsman F, et al. Autoimmune polyendocrine syndrome type 1 (APS I) in Norway. Clin Endocrinol (Oxf) 2001 Feb;54(2):211-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11207636
(61) Petite J, Rosset N, Chapuis B, Jeannet M. Genetic factors predisposing to autoimmune diseases. Study of HLA antigens in a family with pernicious anemia and thyroid diseases. Schweiz Med Wochenschr 1987;117(50):2032-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3433088
(62) Cavadini P, Vermi W, Facchetti F, Fontana S, Nagafuchi S, Mazzolari E, et al. AIRE deficiency in thymus of 2 patients with Omenn syndrome. J Clin Invest 2005 Mar;115(3):728-32.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15696198
(63) Halonen M, Eskelin P, Myhre AG, Perheentupa J, Husebye ES, Kampe O, et al. AIRE Mutations and Human Leukocyte Antigen Genotypes as Determinants of the Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy Phenotype. J Clin Endocrinol Metab 2002 Jun;87(6):2568-74.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12050215
(64) Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet 2002 Feb 15;11(4):397-409.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11854172
(65) Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002 Nov 15;298(5597):1395-401.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12376594
(66) Hanahan D. Peripheral-antigen-expressing cells in thymic medulla: factors in self- tolerance and autoimmunity. Curr Opin Immunol 1998 Dec;10(6):656-62.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9914224
(67) Heath VL, Moore NC, Parnell SM, Mason DW. Intrathymic expression of genes involved in organ specific autoimmune disease. J Autoimmun 1998 Aug;11(4):309-18.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9776708
(68) Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 1997;15:289-92.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9054944
(69) Pugliese A, Zeller M, Fernandez A, Zalcberg LJ, Bartlett RJ, Ricordi C, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type I diabetes. Nat Genet 1997;15(3):293-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9054945
(70) Chentoufi AA, Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. diab 2002 May;51(5):1383-90.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11978634
(71) Pugliese A, Miceli D. The insulin gene in diabetes. Diabetes Metab Res Rev 2002 Jan;18(1):13-25.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11921414
(72) Pugliese A. Peripheral antigen-expressing cells and autoimmunity. Endocrinology and Metabolism Clinics of North America. 31 ed. W.B. Saunders; 2002. p. 411-30.
(73) Taubert R, Schwendemann J, Kyewski B. Highly variable expression of tissue-restricted self-antigens in human thymus: Implications for self-tolerance and autoimmunity. Eur J Immunol 2007 Mar;37(3):838-48.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17323415&query_hl=1&itool=pubmed_docsum
(74) Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005 May 12;435(7039):220-3.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=15889095
(75) Nakayama M, Beilke JN, Jasinski JM, Kobayashi M, Miao D, Li M, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest 2007 Jul 2;117(7):1835-43.http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=17607359&ordinalpos=20&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(76) Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 2006 Dec 1;116(12):3258-65.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17143333&query_hl=7&itool=pubmed_docsum
(77) Krishnamurthy B, Mariana L, Gellert SA, Colman PG, Harrison LC, Lew AM, et al. Autoimmunity to Both Proinsulin and IGRP Is Required for Diabetes in Nonobese Diabetic 8.3 TCR Transgenic Mice. J Immunol 2008 Apr 1;180(7):4458-64.http://www.ncbi.nlm.nih.gov/pubmed/18354167?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(78) Thebault-Baumont K, Dubois-LaForgue D, Krief P, Briand JP, Halbout P, Vallon-Geoffroy K, et al. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest 2003 Mar 15;111(6):851-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12639991
(79) Elbein SC, Hoffman MD, Mayorga RA, Barrett KL, Leppert M, Hasstedt S. Do non-insulin dependent diabetes mellitus (NIDDM) and insulin-dependent diabetes mellitus (IDDM) share genetic susceptibility loci? An analysis of putative IDDM susceptibility regions in familial NIDDM. metab 1997;46(1):48-52.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9005968
(80) Villasenor J, Benoist C, Mathis D. AIRE and APECED: molecular insights into an autoimmune disease. Immunol Rev 2005 Apr;204:156-64.:156-64.http://www.ncbi.nlm.nih.gov/pubmed/15790357?ordinalpos=4&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(81) Pugliese A, Brown D, Garza D, Zeller M, Redondo MJ, Eisenbarth GS, et al. Self-Antigen Presenting Cells Expressing Islet Cell Molecules in Human Thymus and Peripheral Lymphoid Organs: Phenotypic Characterization and Implications for Immunological Tolerance and Type 1 Diabetes. J Clin Invest 2001;107(5):555-64.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11238556
(82) Gardner JM, DeVoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 2008 Aug 8;321(5890):843-7.PM:18687966
(83) Metzger TC, Anderson MS. Control of central and peripheral tolerance by Aire. Immunol Rev 2011 May;241(1):89-103.PM:21488892
(84) Cetani F, Barbesino G, Borsari S, Pardi E, Cianferotti L, Pinchera A, et al. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab 2001 Oct;86(10):4747-52.http://www.ncbi.nlm.nih.gov/pubmed/11600535?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(85) Perheentupa J, Miettinen A. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. In: Eisenbarth GS, editor. Endocrine and Organ Specific Autoimmunity.Austin: R.G. Landes Company; 1999. p. 19-40.
(86) De Bellis A, Bizzarro A, Rossi R, Amoresano Paglionico V, Criscuolo T, Lombardi G, et al. Remission of subclinical adrenocortical failure in subjects with adrenal autoantibodies. J Clin Endocrinol Metab 1993;76:1002-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8473373
(87) Clemente MG, Obermayer-Straub P, Meloni A, Strassburg CP, Arangino V, Tukey RH, et al. Cytochrome P450 1A2 is a hepatic autoantigen in autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab 1997;82(5):1353-61.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9141515
(88) Radetti G, Paganini C, Gentili L, Bernasconi S, Beterle C, Borkenstein M, et al. Frequency of Hashimoto's thyroiditis in children with type 1 diabetes mellitus. Acta Diabetol 1995;32:121-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7579533
(89) Landin-Olsson M, Karlsson FA, Lernmark Å, Sundkvist G, and the Diabetes Incidence Study in Sweden Group. Islet cell and thyrogastric antibodies in 633 consecutive 15- to 34-yr-old patients in the diabetes incidence study in Sweden. diab 1992;41:1022-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1628762
(90) Fairfax AJ, Leatham A. Idiopathic heart block: association with vitiligo, thyroid disease, pernicious anemia, and diabetes mellitus. BMJ 1975;4(5992):322-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1192048
(91) Davis RE, VcCann VJ, Stanton KG. Type 1 diabetes and latent pernicious anaemia. Med J Aust 1992;156(3):160-2.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1545717
(92) Peserico A, Rigon F, Semsenzato G, Caretto A, Pasini CV, Betterle C. Vitiligo and polyglandular autoimmune disease with autoantibodies to melanin-producing cells. A new syndrome? Arch Dermatol 1981;117(11):751-2.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7316541
(93) Betterle C, Caretto A, Pedini B, Rigon F, Bertoli P, Peserico A. Complement-fixing activity to melanin-producing cells preceding the onset of vitiligo in a patient with type I polyglandular failure. Arch Dermatol 1995;128:123-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1739282
(94) Torrelo A, España A, Balsa J, Ledo A. Vitiligo and polyglandular autoimmune syndrome with selective IgA deficiency. Int J Dermatol 1992;31(5):343-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1587664
(95) Stankler L, Bewsher PD. Chronic mucocutaneous candidiasis endocrine deficiency and alopecia areata. Br J Dermatol 1972;86(3):238-45.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=5018678
(96) Hedstrand H, Perheentupa J, Ekwall O, Gustafsson J, Michaelsson G, Husebye E, et al. Antibodies against hair follicles are associated with alopecia totalis in autoimmune polyendocrine syndrome type I. J Invest Dermatol 1999 Dec;113(6):1054-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10594751
(97) Bosch EP, Reith PE, Granner DK. Myasthenia gravis and Schmidt syndrome. Semin Neurol 1994;27:1179-80.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=563019
(98) Marino M, Ricciardi R, Pinchera A, Barbesino G, Manetti L, Chiovato L, et al. Mild clinical expression of myasthenia gravis associated with autoimmune thyroid diseases. J Clin Endocrinol Metab 1997;82(2):438-43.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9024233
(99) Collin P, Salmi J, Hällström O, Reunala T, Pasternack A. Autoimmune thyroid disorders and coeliac disease. Eur J Endocrinol 1994;130:137-40.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8130887
(100) Reijonen H, Ilonen J, Knip M, Reunala T. Insulin-dependent diabetes mellitus associated with dermatitis herpetiformis: evidence for heterogeneity of HLA-associated genes. Tissue Antigens 1991;37:94-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2057939
(101) Reunala T, Salmi J, Karvonen J. Dermatitis herpetiformis and celiac disease associated with Addison's disease. Arch Dermatol 1987;123:930-2.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3606172
(102) Tucker WSJr, Niblack GD, McLean RH, Alspaugh MA, Wyatt RJ, Jordan SC, et al. Serositis with autoimmune endocrinopathy: clinical and immunogenetic features. Medicine 1987;66(2):138-47.http://www.ncbi.nlm.nih.gov/pubmed/3493414?ordinalpos=20&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(103) Merenmies L, Tarkkanen A. Chronic bilateral keratitis in autoimmune polyendocrinopathy-candidiadis-ectodermal dystrophy (APECED). A long-term follow-up and visual prognosis [In Process Citation]. Acta Ophthalmol Scand 2000 Oct;78(5):532-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11037909
(104) Turkington RW, Lebovitz HE. Extra-adrenal endocrine deficiencies in Addison's disease. Am J Med 1967;43:499-507.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=4168683
(105) Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med 1990;322(22):1555-60.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2135382
(106) Selinger S, Tsai J, Pulini M, Saperstein A, Taylor S. Autoimmune thrombocytopenia and primary billary cirrhosis with hypoglycemia and insulin receptor autoantibodies. Ann Intern Med 1987;107:686-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3310794
(107) Rhim SH, Millar SE, Robey F, Luo AM, Lou YH, Yule T, et al. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J Clin Invest 1992;89:28-35.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1370297
(108) Muir A, Maclaren NK. Autoimmune diseases of the adrenal glands, parathyroid glands, gonads, and hypothalamic-pituitary axis. Endocrinol Metab Clin North Am 1991;20:619-44.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1935921
(109) Barkan AL, Kelch RP, Marshall JC. Isolated gonadotrope failure in the polyglandular autoimmune syndrome. N Engl J Med 1985;312:1535-40.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3923349
(110) Petersen JS, Dyrberg T, Karlsen AE, Molvig J, Michelsen B, Nerup J, et al. Glutamic acid decarboxylase (GAD65) autoantibodies in prediction of B-cell function and remission in recent-onset IDDM after cyclosporin treatment. diab 1994;43(11):1291-6.http://www.ncbi.nlm.nih.gov/pubmed/7926302?ordinalpos=22&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(111) Efthimiou P, Flavell RA, Furlan A, Gasbarrini G, Gava A, Kone-Paut I, et al. Autoinflammatory syndromes and infections: pathogenetic and clinical implications. Clin Exp Rheumatol 2008 Jan;26(1 Suppl 48):S53-S61.http://www.ncbi.nlm.nih.gov/pubmed/18570755?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(112) Martinon F. Detection of immune danger signals by NALP3. J Leukoc Biol 2008 Mar;83(3):507-11.http://www.ncbi.nlm.nih.gov/pubmed/17982111?ordinalpos=4&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(113) Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 2007 Dec;19(6):615-22.http://www.ncbi.nlm.nih.gov/pubmed/17977705?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(114) Pope RM, Tschopp J. The role of interleukin-1 and the inflammasome in gout: implications for therapy. Arthritis Rheum 2007 Oct;56(10):3183-8.http://www.ncbi.nlm.nih.gov/pubmed/17907163?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(115) Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008 Jun;453(7198):1122-6.http://www.ncbi.nlm.nih.gov/pubmed/18496530?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(116) Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. J Dermatol Sci 2006 Jan;41(1):3-10.http://www.ncbi.nlm.nih.gov/pubmed/17630960?ordinalpos=5&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(117) Soderbergh A, Rorsman F, Halonen M, Ekwall O, Bjorses P, Kampe O, et al. Autoantibodies against aromatic L-amino acid decarboxylase identifies a subgroup of patients with Addison's disease. J Clin Endocrinol Metab 2000 Jan;85(1):460-3.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10634424
(118) Gylling M, Tuomi T, Bjorses P, Kontiainen S, Partanen J, Christie MR, et al. ss-Cell Autoantibodies, Human Leukocyte Antigen II Alleles, and Type 1 Diabetes in Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. J Clin Endocrinol Metab 2000 Dec;85(12):4434-40.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11134089
(119) Ekwall O, Hedstrand H, Haavik J, Perheentupa J, Betterle C, Gustafsson J, et al. Pteridin-dependent hydroxylases as autoantigens in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab 2000 Aug;85(8):2944-50.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10946908
(120) Perniola R, Falorni A, Clemente MG, Forini F, Accogli E, Lobreglio G. Organ-specific and non-organ-specific autoantibodies in children and young adults with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) [In Process Citation]. Eur J Endocrinol 2000 Oct;143(4):497-503.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11022196
(121) Soderbergh A, Myhre AG, Ekwall O, Gebre-Medhin G, Hedstrand H, Landgren E, et al. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab 2004 Feb;89(2):557-62.http://www.ncbi.nlm.nih.gov/pubmed/14764761?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(122) Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol 2008 Jan 4;.http://www.ncbi.nlm.nih.gov/pubmed/18178393?ordinalpos=28&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(123) Perheentupa J. APS-I/APECED: The clinical disease and therapy. In: Eisenbarth GS, editor. Autoimmune Polyendocrine Syndromes.Philadelphia: W.B. Saunders Company; 2002. p. 295-320.
(124) Walker DA, Davies M. Addison's disease presenting as a hypercalcemic crisis in a patient with idiopathic hypoparathyroidism. Clin Endocrinol (Oxf) 1981 Apr;14(4):419-23.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7261421
(125) Ahonen P, Myllarniemi S, Kahanpaa A, Perheentupa J. Ketoconazole is effective against the chronic mucocutaneous candidosis of autoimmune polyendocrinopathy-candidosis-ectodermal dystrophy (APECED). Acta Med Scand 1986;220(4):333-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3541501
(126) Starzyk J, Kumorowicz-Kopiec M, Kowalczyk M, Starzyk B, Rybakowa M, Dziatkowiak H. Natural history of asplenism in APECED--patient report. J Pediatr Endocrinol Metab 2001 Apr;14(4):443-9.http://www.ncbi.nlm.nih.gov/pubmed/11327379?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(127) Betterle C, Volpato M, Greggio AN, Presotto F. Type 2 polyglandular autoimmune disease ISchmitd's syndrome). J Pediatr Endocrinol Metab 1996;9:113-23.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8887161
(128) Savilahti E, Simell O, Koskimies S, Rilva A, Åkerblom HK. Celiac disease in insulin-dependent diabetes mellitus. J Pediatr 1986;108(5 Pt 1):690-3.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3701514
(129) Bao F, Yu L, Babu S, Wang T, Hoffenberg EJ, Rewers M, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease associated transglutaminase autoantibodies. J Autoimmunity 1999;13:143-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10441179
(130) Papadopoulos KI, Hornblad Y, Hallengren B. The occurrence of polyglandular autoimmune syndrome type III associated with coeliac disease in patients with sarcoidosis. J Intern Med 1994;236:661-3.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7989901
(131) Bellastella G, Rotondi M, Pane E, Dello IA, Pirali B, Dalla ML, et al. Predictive role of the immunostaining pattern of immunofluorescence and the titers of antipituitary antibodies at presentation for the occurrence of autoimmune hypopituitarism in patients with autoimmune polyendocrine syndromes over a five-year follow-up. J Clin Endocrinol Metab 2010 Aug;95(8):3750-7.PM:20501686
(132) Papathanasiou A, Kousta E, Skarpa V, Papachileos P, Petrou V, Hadjiathanasiou C. Growth hormone deficiency in a patient with autoimmune polyendocrinopathy type 2. Hormones (Athens ) 2007 Jul;6(3):247-50
(133) Furmaniak J, Sanders J, Rees Smith B. Autoantigens in the autoimmune endocrinopathies. In: Volpe R, editor. Contemporary Endocrinology: Autoimmune Endocrinopathies.Totowa: Humana Press Inc.; 1999. p. 183-216.
(134) Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 2007 Jul;39(7):857-64.http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=17554260&ordinalpos=6&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(135) Baker P, Fain P, Kahles H, Yu L, Hutton J, Wenzlau J, et al. Genetic determinants of 21-hydroxylase autoantibodies amongst patients of the type 1 diabetes genetics consortium. J Clin Endocrinol Metab 2012 Aug;97(8):E1573-E1578.PM:22723331
(136) Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 2004 Apr;36(4):337-8.http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15004560
(137) Skinningsrud B, Husebye ES, Gervin K, Lovas K, Blomhoff A, Wolff AB, et al. Mutation screening of PTPN22: association of the 1858T-allele with Addison's disease. Eur J Hum Genet 2008 Feb 27;.16(8):977-82.http://www.ncbi.nlm.nih.gov/pubmed/18301444?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(138) Lee YH, Rho YH, Choi SJ, Ji JD, Song GG, Nath SK, et al. The PTPN22 C1858T functional polymorphism and autoimmune diseases--a meta-analysis. Rheumatology (Oxford) 2007 Jan;46(1):49-56.http://www.ncbi.nlm.nih.gov/pubmed/16760194?ordinalpos=5&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(139) Barker JM. Clinical review: Type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab 2006 Apr;91(4):1210-7.PM:16403820
(140) Kahles H, Ramos-Lopez E, Lange B, Zwermann O, Reincke M, Badenhoop K. Sex-specific association of PTPN22 1858T with type 1 diabetes but not with Hashimoto's thyroiditis or Addison's disease in the German population. Eur J Endocrinol 2005 Dec;153(6):895-9.http://www.ncbi.nlm.nih.gov/pubmed/16322396?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(141) Perez dN, Martin-Pagola A, Ramon BJ, Vazquez F, Castano L. No evidence of association of CTLA4 polymorphisms with Addison's disease. Autoimmunity 2004 Sep;37(6-7):453-6
(142) Blomhoff A, Lie BA, Myhre AG, Kemp EH, Weetman AP, Akselsen HE, et al. Polymorphisms in the cytotoxic T lymphocyte antigen-4 gene region confer susceptibility to Addison's disease. J Clin Endocrinol Metab 2004 Jul;89(7):3474-6.http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15240634
(143) Donner H, Braun J, Seidl C, Rau H, Finke R, Ventz M, et al. Codon 17 polymorphism of the cytotoxic T lymphocyte antigen 4 gene in Hashimoto's thyroiditis and Addison's disease. J Clin Endocrinol Metab 1997 Dec;82(12):4130-2
(144) Zurawek M, Fichna M, Januszkiewicz D, Fichna P, Nowak J. Polymorphisms in the Interferon-Induced Helicase (IFIH1) locus and susceptibility to Addison's disease. Clin Endocrinol (Oxf) 2012 Jul 12.PM:22789000
(145) Zeitlin AA, Heward JM, Newby PR, Carr-Smith JD, Franklyn JA, Gough SC, et al. Analysis of HLA class II genes in Hashimoto's thyroiditis reveals differences compared to Graves' disease. Genes Immun 2008 Jun;9(4):358-63.http://www.ncbi.nlm.nih.gov/pubmed/18449200?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(146) Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 2007 Nov;39(11):1329-37.http://www.ncbi.nlm.nih.gov/pubmed/17952073?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(147) Jacobson EM, Tomer Y. The genetic basis of thyroid autoimmunity. Thyroid 2007 Oct;17(10):949-61.http://www.ncbi.nlm.nih.gov/pubmed/17824829?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(148) Roycroft M, Fichna M, McDonald D, Owen K, Zurawek M, Gryczynska M, et al. The tryptophan 620 allele of the lymphoid tyrosine phosphatase (PTPN22) gene predisposes to autoimmune Addison's disease. Clin Endocrinol (Oxf) 2009 Mar;70(3):358-62.PM:18710467
(149) Mitchell AL, Cordell HJ, Soemedi R, Owen K, Skinningsrud B, Wolff AB, et al. Programmed death ligand 1 (PD-L1) gene variants contribute to autoimmune Addison's disease and Graves' disease susceptibility. J Clin Endocrinol Metab 2009 Dec;94(12):5139-45.PM:19850680
(150) Lopez ER, Zwermann O, Segni M, Meyer G, Reincke M, Seissler J, et al. A promoter polymorphism of the CYP27B1 gene is associated with Addison's disease, Hashimoto's thyroiditis, Graves' disease and type 1 diabetes mellitus in Germans. Eur J Endocrinol 2004 Aug;151(2):193-7
(151) Pani MA, Seissler J, Usadel KH, Badenhoop K. Vitamin D receptor genotype is associated with Addison's disease. Eur J Endocrinol 2002 Nov;147(5):635-40
(152) Yu L, Brewer KW, Gates S, Wu A, Wang T, Babu S, et al. DRB1*04 and DQ alleles: expression of 21-hydroxylase autoantibodies and risk of progression to Addison's disease. J Clin Endocrinol Metab 1999;84(1):328-35.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9920103
(153) Myhre AG, Undlien DE, Lovas K, Uhlving S, Nedrebo BG, Kristian JF, et al. Autoimmune adrenocortical failure in Norway: Autoantibodies and HLA class II associations related to clinical features. J Clin Endocrinol Metab 2002;87(2):618-23.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11836294
(154) Gombos Z, Hermann R, Kiviniemi M, Nejentsev S, Reimand K, Fadeyev V, et al. Analysis of extended human leukocyte antigen haplotype association with Addison's disease in three populations. Eur J Endocrinol 2007 Dec;157(6):757-61.http://www.ncbi.nlm.nih.gov/pubmed/18057383?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(155) Eisenbarth GS, Wilson PW, Ward F, Buckley C, Lebovitz HE. The polyglandular failure syndrome: disease inheritance, HLA- type and immune function. Ann Intern Med 1979;91(4):528-33.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=314765
(156) Maclaren NK, Riley WJ. Inherited susceptibility to autoimmune Addison's disease is linked to human leukocyte antigens-DR3 and/or DR4, except when associated with type 1 autoimmune polyglandular syndrome. J Clin Endocrinol Metab 1986;62 (3):455-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3484749
(157) Butler MG, Hodes ME, Conneally PM, Biegel AA, Wright JC. Linkage analysis in a large kindred with autosomal dominant transmission of polyglandular autoimmune disease type II (Schmidt syndrome). Am J Med Genet 1984;18:61-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=6588752
(158) Farid NR, Larsen B, Payne R, Noel EP, Sampson L. Polyglandular autoimmune disease and HLA. Tissue Antigens 1980;16:23-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7008251
(159) Valenta LJ, Bull RW, Hackel E, Bottazzo GF. Correlation of the HLA-A1, B8 haplotypes with circulating autoantibodies in a family with increased incidence of autoimmune disease. Acta Endocrinol 1982;100(1):143-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=6981277
(160) Liblau RS, Caillat-Zucman S, Fischer AM, Bach JF, Boitard C. The prevalence of selective IgA deficiency in type 1 diabetes mellitus. Acta Pathol Microbiol Immunol Scand 1992;100:709-12.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1520483
(161) French MAH, Dawkins RL. Central MHC genes, IgA deficiency and autoimmune disease. Immunol Today 1990;11(8):271-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2206270
(162) Alper CA, Marcus-Bagley D, Awdeh Z, Kruskall MS, Eisenbarth GS, Brink SJ, et al. Prospective analysis suggests susceptibility genes for deficiencies of IgA and several other immunoglobulins on the [HLA-B8, SC01, DR3] conserved extended haplotype. Tissue Antigens 2000;56(3):207-16.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11034556
(163) Valenta LJ, Elias AN. Familial scleroderma in a kindred with high incidence of autoimmune disease: correlation with HLA-A1/B8 haplotype. Arch Dermatol 1987;123:1438-40.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3499864
(164) Candrina R, Giustina A. Development of type II autoimmune polyglandular syndrome in a patient with idiopathic thrombocytopenic purpura. Isr J Med Sci 1988;24:57-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3346154
(165) Vela BS, Dorin RI, Hartshorne MF. Case report 631: Neo-osseous porosis (metaphyseal osteopenia) in polyglandular autoimmune (Schmidt) syndrome. Skeletal Radiol 1990;19:468-71.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2218600
(166) Simmonds MJ, Howson JM, Heward JM, Carr-Smith J, Franklyn JA, Todd JA, et al. A novel and major association of HLA-C in Graves' disease that eclipses the classical HLA-DRB1 effect. Hum Mol Genet 2007 Sep 15;16(18):2149-53.http://www.ncbi.nlm.nih.gov/pubmed/17597093?ordinalpos=6&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(167) Bloch MH, Sowers JR. Vitiligo and polyglandular autoimmune endocrinopathy. Cutis 1985;36:417-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=4064762
(168) Inoue D, Sato K, Sugawa H, Akamizu T, Maeda M, Inoko H, et al. Apparent genetic difference between hypothyroid patients with blocking-type thyrotropin receptor antibody and those without, as shown by restriction fragment length polymorphism analyses of HLA-DP loci. J Clin Endocrinol Metab 1993;77(3):606-10.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8103768
(169) Cho BY, Chung JH, Shong YK, Chang YB, Han H, Lee J-B, et al. A strong association between thyrotropin receptor-blocking antibody-positive atrophic autoimmune thyroiditis and HLAL-DR8 and HLA-DQB1*0302 in Koreans. J Clin Endocrinol Metab 1993;77(3):611-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8103769
(170) Santamaria P, Barbosa JJ, Lindstrom AL, Lemke TA, Goetz FC, Rich SS. HLA-DQB1-associated susceptibility that distinguishes Hashimoto's thyroiditis from Graves' disease in type I diabetic patients. J Clin Endocrinol Metab 1994;78(4):878-83.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8157715
(171) Baker PR, Baschal EE, Fain PR, Nanduri P, Triolo TM, Siebert JC, et al. Dominant Suppression of Addison's Disease Associated with HLA-B15. J Clin Endocrinol Metab 2011 May 11;Epub.PM:21565792
(172) Gambelunghe G, Falorni A, Ghaderi M, Laureti S, Tortoioli C, Santeusanio F, et al. Microsatellite Polymorphism of the MHC Class I Chain-related (MIC-A and MIC-B) Genes Marks the Risk for Autoimmune Addison's Disease. J Clin Endocrinol Metab 1999;84:3701-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10523017
(173) Kristiansen OP, Larsen ZM, Pociot F. CTLA-4 in autoimmune diseases--a general susceptibility gene to autoimmunity? Genes Immun 2000 Feb;1(3):170-84.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11196709
(174) Park YS, Sanjeevi CB, Robles D, Yu L, Rewers M, Gottlieb PA, et al. Additional association of intra-MHC genes, MICA and D6S273, with Addison's disease. Tissue Antigens 2002 Aug;60(2):155-63.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12392510
(175) Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005 May 18;293(19):2343-51.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15900004
(176) Drosos AA, Christou L, Galanopoulou V, Tzioufas AG, Tsiakou EK. D-penicillamine induced myasthenia gravis: clinical, serological and genetic findings. Clin Exp Rheumatol 1993;11:387-91.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8403583
(177) Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999 Nov 13;354(9191):1691-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10568572
(178) Schreuder TC, Gelderblom HC, Weegink CJ, Hamann D, Reesink HW, Devries JH, et al. High incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infection. Liver Int 2008 Jan;28(1):39-46.http://www.ncbi.nlm.nih.gov/pubmed/18031478?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(179) Schreuder TC, Gelderblom HC, Weegink CJ, Hamann D, Reesink HW, Devries JH, et al. High incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infection. Liver Int 2007 Nov 21;.http://www.ncbi.nlm.nih.gov/pubmed/18031478?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(180) Soultati AS, Dourakis SP, Alexopoulou A, Deutsch M, Archimandritis AJ. Simultaneous development of diabetic ketoacidosis and Hashitoxicosis in a patient treated with pegylated interferon-alpha for chronic hepatitis C. World J Gastroenterol 2007 Feb 28;13(8):1292-4.http://www.ncbi.nlm.nih.gov/pubmed/17451219?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(181) Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am 2007 Dec;36(4):1051-66.http://www.ncbi.nlm.nih.gov/pubmed/17983936?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(182) Badra G, Waked I, Selmi C, Saleh SM, El Shaarawy A, Lotfy M. Serum islet cell autoantibodies during interferon alpha treatment in patients with HCV-genotype 4 chronic hepatitis. Clin Dev Immunol 2006 Mar;13(1):11-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=16603440&query_hl=20&itool=pubmed_docsum
(183) Krysiak R, Boldys A, Okopien B. Autoimmune polyglandular syndrome type 2 induced by treatment with interferon alpha. Am J Med Sci 2011 Jun;341(6):504-7.PM:21412133
(184) Liu E, Li M, Emery L, Taki I, Barriga K, Tiberti C, et al. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J Pediatr Gastroenterol Nutr 2007 Sep;45(3):293-300.http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=17904423&ordinalpos=16&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(185) Hoffenberg EJ, Haas J, Drescher A, Barnhurst R, Osberg I, Bao F, et al. A trial of oats in newly diagnosed celiac disease. J Pediatr 2000 Sep;137(3):361-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10969261
(186) Green PH, Cellier C. Celiac disease. N Engl J Med 2007 Oct 25;357(17):1731-43
(187) Atkinson MA, Bowman MA, Kao K, Campbell L, Dush PJ, Shah SC, et al. Lack of immune responsiveness to bovine serum albumin in insulin- dependent diabetes. N Engl J Med 1993;329:1853-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8247037
(188) Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007 Sep 26;298(12):1420-8.http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=17895458&ordinalpos=22&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(189) Burman P, Totterman TH, Oberg K, Karlsson FA. Thyroid autoimmunity in patients on long term therapy with leukocyte- derived interferon. J Clin Endocrinol Metab 1986;63:1086-90.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2944910
(190) Papo T, Oksenhendler E, Izembart M, Leger A, Clauvel J-P. Antithyroid hormone antibodies induced by interferon-a. J Clin Endocrinol Metab 1992;75:1484-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1464652
(191) Gisslinger H, Gilly B, Woloszczuk W, Mayr WR, Havelec L, Linkesch W, et al. Thyroid autoimmunity and hypothyroidism during long-term treatment with recombinant interferon-alpha. Clin Exp Immunol 1992;90:363-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1458673
(192) Schilling RF. Who has vitamin B12 deficiency? Proc Assoc Am Physicians 1996;108(1):68-70
(193) Baker PR, Nanduri P, Gottlieb PA, Yu L, Klingensmith GJ, Eisenbarth GS, et al. Predicting the Onset of Addison's Disease: ACTH, Renin, Cortisol, and 21-hydroxylase Autoantibodies. Clin Endocrinol (Oxf) 2011 Nov 8.PM:22066755
(194) de Carmo S, Kater CE, Dib SA, Laureti S, Forini F, Cosentino A, et al. Autoantibodies against recombinant human steroidogenic enzymes 21-hydroxylase, side-chain cleavage and 17alpha-hydroxylase in Addison's disease and autoimmune polyendocrine syndrome type III. Eur J Endocrinol 2000 Feb;142(2):187-94.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10664529
(195) Bosi E, Becker F, Bonifacio E, Wagner R, Collins P, Gale EA, et al. Progression to type I diabetes in autoimmune endocrine patients with islet cell antibodies. diab 1991;40(8):977-84.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1860562
(196) Wagner R, Genovese S, Bosi E, Becker F, Bingley PJ, Bonifacio E, et al. Slow metabolic deterioration towards diabetes in islet cell antibody positive patients with autoimmune polyendocrine disease. diabetol 1994;37:365-71.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8063036
(197) Blizzard RM, Kyle M. Studies of the adrenal antigens and antibodies in Addison's disease. J Clin Invest 1963;42:1653-60.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14074360
(198) Soderbergh A, Winqvist O, Norheim I, Rorsman F, Husebye ES, Dolva O, et al. Adrenal autoantibodies and organ-specific autoimmunity in patients with Addison's disease. Clin Endocrinol (Oxf) 1996;45:453-60.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8959085
(199) Julien JF, Samama P, Mallet J. Rat brain glutamic acid deciarboxylase sequence deduced from a cloned cDNA. J Neurochem 1990;54(2):703-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2299361
(200) Betterle C, Volpato M, Rees Smith B, Furmaniak J, Chen S, Zanchetta R, et al. II. Adrenal cortex and steroid 21-hydroxylase autoantibodies in children with organ-specific autoimmune diseases: markers of high progression to clinical Addison's disease. J Clin Endocrinol Metab 1997;82(3):939-42.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9062510
(201) Tung KSK, Taguchi O, Teuscher C. Testicular and ovarian autoimmune diseases. 1993.
(202) Coco G, Dal Pra C, Presotto F, Albergoni MP, Canova C, Pedini B, et al. Estimated risk for developing autoimmune Addison's disease in patients with adrenal cortex autoantibodies. J Clin Endocrinol Metab 2006 May;91(5):1637-45.http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=16522688&ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(203) Posillico JT, Wortsman J, Srikanta S, Eisenbarth GS, Malette LE, Brown EM. Parathyroid cell surface autoantibodies that inhibit parathyroid hormone secretion from dispersed human parathyroid cells. Bone Miner Res 1986;1(5):475-83.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3332555
(204) Posillico JT, Srikanta S, Eisenbarth G, Quaranta V, Kajiji S, Brown EM. Binding of monoclonal antibody (4F2) to its cell surface antigen on dispersed adenomatous parathyroid cells raises cytosolic calcium and inhibits parathyroid secretion. J Clin Endocrinol Metab 1987;64(1):43-50.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3782435
(205) Harris HE, Kemp EH, Brown EM, Weetman AP, Swaminathan K. First report of anti-calcium-sensing receptor antibodies in a patient with Sjogren's syndrome and primary hypoparathyroidism. Rheumatology (Oxford) 2011 Jun;50(6):1173-5.PM:21454310
(206) Brown EM. Anti-parathyroid and anti-calcium sensing receptor antibodies in autoimmune hypoparathyroidism. Endocrinol Metab Clin North Am 2009 Jun;38(2):437-45, x.PM:19328421
(207) Kemp EH, Gavalas NG, Krohn KJ, Brown EM, Watson PF, Weetman AP. Activating autoantibodies against the calcium-sensing receptor detected in two patients with autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab 2009 Dec;94(12):4749-56.PM:19837919
(208) Bach J-F, Yamamoto AM, Djabiri F, Garchon H-J. Etiopathogenesis of myasthenia gravis (MG). In: Eisenbarth GS, editor. Molecular mechanisms of endocrine and organ specific autoimmunity.Georgetown: Landes Bioscience; 1997.
(209) Sanders J, Oda Y, Roberts S, Kiddie A, Richards T, Bolton J, et al. The interaction of TSH receptor autoantibodies with 125I-labelled TSH receptor. J Clin Endocrinol Metab 1999 Oct;84(10):3797-802.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10523032
(210) Wenzlau JM, Gardner TJ, Frisch LM, Davidson HW, Hutton JC. Development of a novel autoantibody assay for autoimmune gastritis in type 1 diabetic individuals. Diabetes Metab Res Rev 2011 Nov;27(8):887-90.PM:22069279
(211) Karlsson FA, Burman P, Loof L, Mardh S. Major parietal cell antigen in autoimmune gastritis and pernicious anemia is the acid producing HK-ATPase of the stomach. J Clin Invest 1988;81:475-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2828428
(212) De Block CE, De L, I, Van Gaal LF. High prevalence of manifestations of gastric autoimmunity in parietal cell antibody-positive type 1 (insulin-dependent) diabetic patients. The Belgian Diabetes Registry. J Clin Endocrinol Metab 1999 Nov;84(11):4062-7
(213) Bjork E, Velloso LA, Kampe O, Karlsson FA. GAD autoantibodies in IDDM, stiff-man syndrome, and autoimmune polyendocrine syndrome type I recognize different epitopes. Diabetes 1994 Jan;43(1):161-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7505244
(214) Liu E, Eisenbarth GS. Accepting clocks that tell time poorly: fluid-phase versus standard ELISA autoantibody assays. Clin Immunol 2007 Nov;125(2):120-6.http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17904423
(215) Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007 Oct 23;104(43):17040-5.http://www.ncbi.nlm.nih.gov/pubmed/17942684?ordinalpos=8&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(216) Takasu N, Yamada T, Takasu M, Komiya I, Nagasawa Y, Asawa T, et al. Disappearance of thyrotropin-blocking antibodies and spontaneous recovery from hypothyroidism in autoimmune thyroiditis. N Engl J Med 1992;326(8):513-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1732791
(217) Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, et al. Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J Autoimmun 1996;9(3):379-83.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8816974
(218) Kita M, Ahmad L, Marians RC, Vlase H, Unger P, Graves PN, et al. Regulation and transfer of a murine model of thyrotropin receptor antibody mediated Graves' disease. Endocrinology 1999 Mar;140(3):1392-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10067867
(219) Hirata Y, Ishizu H. Elevated insulin-binding capacity of serum proteins in a case of spontaneous hypoglycemia and mild diabetes not treated with insulin. Tohoku J Exp Med 1972;107:277-86.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=4118173
(220) Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009 Nov 26;361(22):2143-52.PM:19940299
(221) Rottembourg D, Deal C, Lambert M, Mallone R, Carel JC, Lacroix A, et al. 21-Hydroxylase epitopes are targeted by CD8 T cells in autoimmune Addison's disease. J Autoimmun 2010 Aug 3.PM:20685079
(222) Abiru N, Maniatis AK, Yu L, Miao D, Moriyama H, Wegmann D, et al. Peptide and MHC specific breaking of humoral tolerance to native insulin with the B:9-23 peptide in diabetes prone and normal mice. diab 2001 Jun;50:1274-81.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11375327
(223) Cohen IR, Young DB. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today 1991;105:105-10.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2059311
(224) Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. Journal of Clinical Investigation 2001;108(8):1097-104.http://www.ncbi.nlm.nih.gov/pubmed/11602615?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(225) Zhang Z-G, Wall JR, Bernard NF. Tissue distribution and quantitation of a gene expressing a 64-kDa antigen associated with thyroid-associated ophthalmopathy. Clin Immunol Immunopathol 1996;80:236-44.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8811043
(226) Stumbles PA, Penhale WJ. IDDM in rats induced by thymectomy and irradiation. diab 1993;42:571-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8454108
(227) Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003 Feb 14;299(5609):1057-61.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12522256
(228) Sakaguchi S. Policing the regulators. Nat Immunol 2001 Apr;2(4):283-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11276194
(229) Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995 Aug 1;155(3):1151-64.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7636184
(230) Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med 1985 Jan 1;161(1):72-87.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3871469
(231) Ramanathan S, Bihoreau MT, Paterson AD, Marandi L, Gauguier D, Poussier P. Thymectomy and radiation-induced type 1 diabetes in nonlymphopenic BB rats. diab 2002 Oct;51(10):2975-81.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12351436
(232) Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and. J Immunol 2000 Sep 15;165(6):3105-10.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10975823
(233) Rubio-Cabezas O, Minton JA, Caswell R, Shield JP, Deiss D, Sumnik Z, et al. Clinical heterogeneity in patients with FOXP3 mutations presenting with permanent neonatal diabetes. Diab care 2009 Jan;32(1):111-6.PM:18931102
(234) Matsuki K, Juji T, Tokunaga K, Takamizawa M, Maeda H, Soda M, et al. HLA antigens in japanese patients with myasthenia gravis. J Clin Invest 1990;392:399.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1974553
(235) Garlepp MI, Dawkins RL, Christiansen FT. HLA antigens and acetylcholine receptor antibodies in penicillamine induced myasthenia gravis. BMJ 1983;286(6375):1442-3.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=6404496
(236) Nagvekar N, Moody AM, Moss P, Roxanis I, Curnow J, Beeson D, et al. A pathogenetic role for the thymoma in myasthenia gravis. Autosensitization of IL-4- producing T cell clones recognizing extracellular acetylcholine receptor epitopes presented by minority class II isotypes. J Clin Invest 1998 May 15;101(10):2268-77.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9593783
(237) Taylor SI, Grunberger G, Marcus-Samuels B, Underhill LH, Dons RF, Ryan J, et al. Hypoglycemia associated with antibodies to the insulin receptor. N Engl J Med 1982;307:1422-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7133096
(238) Dons RF, Havlik R, Taylor SI, Baird KL, Chernick SS, Gorden P. Clinical disorders associated with autoantibodies to the insulin receptor. J Clin Invest 1992.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=6350362
(239) Uchigata Y, Kuwata S, Tsushima T, Tokunaga K, Miyamoto M, Tsuchikawa K, et al. Patients with Graves' disease who developed insulin autoimmune syndrome (Hirata disease) possess HLA-Bw62/Cw4/DR4 carrying DRB1*0406. J Clin Endocrinol Metab 1993;77:249-54.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8325948
(240) Mcevoy RC, Fedun B, Cooper LZ, Thomas NM, Rodriguez de Cordoba S, Rubinstein P, et al. Children at high risk of diabetes mellitus: New York studies of families with diabetes and of children with congenital rubella syndrome. Adv Exp Med Biol 1988;246:221-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3074654
(241) Rabinowe SL, Larsen PR, Antman EM, George KL, Friedman PL, Jackson RA, et al. Amiodarone therapy and autoimmune thyroid disease. Increase in a new monoclonal antibody-defined T cell subset. Am J Med 1986;81(1):53-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3487980
(242) Shi Y, Zou M, Robb D, Farid NR. Typing for major histocompatibility complex class II antigens in thyroid tissue blocks: association of Hashimoto's thyroiditis with HLA-DQA0301 and DQB0201 alleles. J Clin Endocrinol Metab 1992;75:943-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1517390
(243) Tomer Y, Barbesino G, Greenberg DA, Concepcion E, Davies TF. Mapping the major susceptibility loci for familial Graves' and Hashimoto's diseases: evidence for genetic heterogeneity and gene interactions. J Clin Endocrinol Metab 1999 Dec;84(12):4656-64.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10599734
(244) Badenhoop K, Walfish PG, Rau H, Fischer S, Nicolay A, Bogner U, et al. Susceptibility and resistance alleles of human leukocyte antigen (HLA) DQA1 and HLA DQB1 are shared in endocrine autoimmune disease. J Clin Endocrinol Metab 1995 Jul;80(7):2112-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7608264
(245) De Block CE. [Diabetes mellitus type 1 and associated organ-specific autoimmunity]. Verh K Acad Geneeskd Belg 2000;62(4):285-328.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11004907
(246) Naughton GK, Eisenger M, Bystryn J-C. Detection of antibodies to melanocytes in vitiligo by specific immunoprecipitation. J Invest Dermatol 1983;81(6):540-2.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=6196421
(247) Xie Z, Chen D, Jiao D, Bystryn JC. Vitiligo antibodies are not directed to tyrosinase. Arch Dermatol 1999 Apr;135(4):417-22.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10206048
(248) Nicholson LB, Wong FS, Ewins DL, Butler J, Holland A, Denaine AG, et al. Susceptibility to autoimmune thyroiditis in Down's syndrome is associated with the major histocompatibility complex class II DQA*0301 allele. Clin Endocrinol 1994;41:381-3.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7955446
(249) Larizza D, Bianchi MM, Lorini R, Maghnie M, Dugoujon JM, Belvedere CM, et al. Autoimmunity, HLA, Gm and Km polymorphism in Turner's syndrome. Autoimmunity 1989;4:69-78.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2491644
(250) Failla P, Ruberto C, Pagano MC, Lombardo M, Bottaro G, Perichon B, et al. Celiac disease in Down's syndrome with HLA serological and molecular studies. J Pediatr Gastroenterol Nutr 1996;23:303-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8890082
(251) Fleming S, Cowell C, Bailey J, Burrow GN. Hashimoto's disease in Turner's syndrome. Clin Invest Med 1988;11(4):243-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3168347
(252) Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective Suppressor Function of Human CD4+ CD25+ Regulatory T Cells in Autoimmune Polyglandular Syndrome Type II. J Exp Med 2004 May 3;199(9):1285-91.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15117972
(253) Martin AM, Maxson MN, Leif J, Mordes JP, Greiner DL, Blankenhorn EP. Diabetes-prone and diabetes-resistant BB rats share a common major diabetes susceptibility locus, iddm4: additional evidence for a "universal autoimmunity locus" on rat chromosome 4. diab 1999 Nov;48(11):2138-44.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10535446
(254) Schranz DB, Lernmark A. Immunology in diabetes: an update. Diabetes Metab Rev 1998 Mar;14(1):3-29.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9605628
(255) Petterson A, Jacob H, Lernmark Å. Lessons from the animal models: the BB rat. In: Palmer JP, editor. Prediction, prevention, and genetic counseling in IDDM.Chichester, England: Wiley; 1996. p. 181-201.
(256) Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol 2001 Sep;2(9):816-22.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11526392
(257) Olsen NJ, Brooks RH, Cush JJ, Lipsky PE, St Clair EW, Matteson EL, et al. A double-blind, placebo-controlled study of anti-CD5 immunoconjugate in patients with rheumatoid arthritis. The Xoma RA Investigator Group. Arthritis Rheum 1996 Jul;39(7):1102-8.http://www.ncbi.nlm.nih.gov/pubmed/8670317?ordinalpos=21&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(258) Wildin RS, Freitas A. IPEX and FOXP3: Clinical and research perspectives. J Autoimmun 2005;25 Suppl:56-62.http://www.ncbi.nlm.nih.gov/pubmed/16243487?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(259) Jackson RA, Haynes BF, Burch WM, Shimizu K, Bowring MA, Eisenbarth GS. Ia+ T cells in new onset Graves' disease. J Clin Endocrinol Metab 1984;59(2):187-90.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=6610685
(260) Kaye WA, Adri MN, Soeldner JS, Kaldany A, Rabinowe SL, Kahn CR, et al. Acquired defect in interleukin-2 production in patients with type I diabetes mellitus. N Engl J Med 1986;315(15):920-4.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3531850
(261) Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, et al. Extreme Th1 bias of invariant Va24JaQ T cells in type 1 diabetes. Nature 1998;391:177-81.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9428763
(262) Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest 2002 Sep;110(6):793-800.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12235110
(263) Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab 2003 Jul;88(7):2983-92.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12843130
(264) Tursi A, Brandimarte G, Giorgetti GM. TI - Prevalence of antitissue transglutaminase antibodies in different degrees of intestinal damage in celiac disease.
(265) Freemark M, Levitsky LL. Screening for Celiac Disease in Children With Type 1 Diabetes: Two views of the controversy. Diab care 2003 Jun;26(6):1932-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12766138
(266) Cronin CC, Feighery A, Ferriss JB, Liddy C, Shanahan F, Feighery C. High prevalence of celiac disease among patients with insulin-dependent (type I) diabetes mellitus. Am J Gastroenterol 1997 Dec;92(12):2210-2.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9399754
(267) Not T, Tommasini A, Tonini G, Buratti E, Pocecco M, Tortul C, et al. Undiagnosed coeliac disease and risk of autoimmune disorders in subjects with Type I diabetes mellitus. diabetol 2001 Feb;44(2):151-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11270670
(268) Liu E, Li M, Bao F, Miao D, Rewers MJ, Eisenbarth GS, et al. Need for quantitative assessment of transglutaminase autoantibodies for celiac disease in screening-identified children. J Pediatr 2005 Apr;146(4):494-9
(269) De BA, Pane E, Bellastella G, Sinisi AA, Colella C, Giordano R, et al. Detection of antipituitary and antihypothalamus antibodies to investigate the role of pituitary or hypothalamic autoimmunity in patients with selective idiopathic hypopituitarism. Clin Endocrinol (Oxf) 2011 Sep;75(3):361-6.PM:21521324
(270) Lupi I, Manetti L, Raffaelli V, Lombardi M, Cosottini M, Iannelli A, et al. Diagnosis and Treatment of Autoimmune Hypophysitis: a Short Review. J Endocrinol Invest 2011 Jul 12.PM:21750396
(271) Leporati P, Landek-Salgado MA, Lupi I, Chiovato L, Caturegli P. IgG4-related hypophysitis: a new addition to the hypophysitis spectrum. J Clin Endocrinol Metab 2011 Jul;96(7):1971-80.PM:21593109
(272) Yamamoto M, Iguchi G, Takeno R, Okimura Y, Sano T, Takahashi M, et al. Adult combined GH, prolactin, and TSH deficiency associated with circulating PIT-1 antibody in humans. J Clin Invest 2011 Jan;121(1):113-9.PM:21123951
(273) Oelkers W, Diederich S, Bahr V. Therapeutic strategies in adrenal insufficiency. Ann Endocrinol (Paris) 2001 Apr;62(2):212-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11353897
(274) De Bellis AA, Falorni A, Laureti S, Perrino S, Coronella C, Forini F, et al. Time course of 21-hydroxylase antibodies and long-term remission of subclinical autoimmune adrenalitis after corticosteroid therapy: case report. J Clin Endocrinol Metab 2001 Feb;86(2):675-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11158030
(275) Yu L, Herold K, Krause-Steinrauf H, McGee PL, Bundy B, Pugliese A, et al. Rituximab Selectively Suppresses Specific Islet Antibodies. diab 2011 Aug 10.PM:21831969
(276) Lachin JM, McGee PL, Greenbaum CJ, Palmer J, Pescovitz MD, Gottlieb P, et al. Sample size requirements for studies of treatment effects on beta-cell function in newly diagnosed type 1 diabetes. PLoS ONE 2011;6(11):e26471.PM:22102862
(277) Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 2007 Dec;117(12):3857-67.http://www.ncbi.nlm.nih.gov/pubmed/18060033?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(278) El Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedus L. B lymphocyte depletion with the monoclonal antibody rituximab in Graves' disease: a controlled pilot study. J Clin Endocrinol Metab 2007 May;92(5):1769-72.http://www.ncbi.nlm.nih.gov/pubmed/17284622?ordinalpos=6&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(279) Salvi M, Vannucchi G, Campi I, Curro N, Dazzi D, Simonetta S, et al. Treatment of Graves' disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol 2007 Jan;156(1):33-40.http://www.ncbi.nlm.nih.gov/pubmed/17218723?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(280) El Fassi D, Nielsen CH, Kjeldsen J, Clemmensen O, Hegedus L. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves' disease. Gut 2008 May;57(5):714-5.http://www.ncbi.nlm.nih.gov/pubmed/18408106?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(281) Bresson D, Togher L, Rodrigo E, Chen YL, Bluestone JA, Herold KC, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. Journal of Clinical Investigation 2006;116(5):1371-81.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=retrieve&db=pubmed&list_uids=16628253&dopt=Abstract
(282) Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8 T cell population and induces CD8CD25 Tregs. J Clin Invest 2005 Oct 1;115(10):2904-13.http://www.ncbi.nlm.nih.gov/pubmed/16167085?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(283) Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1(Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. diab 2005 Jun;54(6):1763-9.http://www.ncbi.nlm.nih.gov/pubmed/15919798?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(284) Herold KC, Hagopian W, Auger J, Gitelman S, Masharani U, Donaldson D, et al. Treatment with Anti-CD3 monoclonal antibody arrests the loss of insulin responses and results in improves metabolic control in pts with type 1 diabetes (T1DM). diab 2002;51:A45.<Go to ISI>://000175934600183
(285) Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005 Jun 23;352(25):2598-608.http://www.ncbi.nlm.nih.gov/pubmed/15972866?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(286) Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol 2007 Aug;7(8):622-32.http://www.ncbi.nlm.nih.gov/pubmed/17641665?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(287) Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol 2008 Mar;9(3):239-44.http://www.ncbi.nlm.nih.gov/pubmed/18285775?ordinalpos=6&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(288) Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol 2005 Sep 1;175(5):3053-9.http://www.ncbi.nlm.nih.gov/pubmed/16116193?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(289) Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest 2006 Jun;116(6):1713-22.http://www.ncbi.nlm.nih.gov/pubmed/16741580?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(290) Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003 Apr;4(4):330-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12612578
(291) Mazzolari E, Forino C, Fontana M, D'Ippolito C, Lanfranchi A, Gambineri E, et al. A new case of IPEX receiving bone marrow transplantation. Bone Marrow Transplant 2005 Mar 21;.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15778724
(292) Zhan H, Sinclair J, Adams S, Cale CM, Murch S, Perroni L, et al. Immune reconstitution and recovery of FOXP3 (forkhead box P3)-expressing T cells after transplantation for IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome. Pediatrics 2008 Apr;121(4):e998-1002.http://www.ncbi.nlm.nih.gov/pubmed/18316354?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(293) Rao A, Kamani N, Filipovich A, Lee SM, Davies SM, Dalal J, et al. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood 2007 Jan 1;109(1):383-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=16990602&query_hl=3&itool=pubmed_docsum
(294) Bindl L, Torgerson T, Perroni L, Youssef N, Ochs HD, Goulet O, et al. Successful use of the new immune-suppressor sirolimus in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome). J Pediatr 2005 Aug;147(2):256-9.http://www.ncbi.nlm.nih.gov/pubmed/16126062?ordinalpos=13&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(295) Rosenstein ED, Advani S, Reitz RE, Kramer N. The prevalence of insulin receptor antibodies in patients with systemic lupus erythematosus and related conditions. J Clin Rheumatol 2001 Dec;7(6):371-3.http://www.ncbi.nlm.nih.gov/pubmed/17039177?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(296) Kato Y, Ichiki Y, Kitajima Y. A case of systemic lupus erythematosus presenting as hypoglycemia due to anti-insulin receptor antibodies. Rheumatol Int 2008 May 24;.http://www.ncbi.nlm.nih.gov/pubmed/18500459?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(297) Page KA, Dejardin S, Kahn CR, Kulkarni RN, Herold KC, Inzucchi SE. A patient with type B insulin resistance syndrome, responsive to immune therapy. Nat Clin Pract Endocrinol Metab 2007 Dec;3(12):835-40.http://www.ncbi.nlm.nih.gov/pubmed/18026162?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(298) Malek R, Chong AY, Lupsa BC, Lungu AO, Cochran EK, Soos MA, et al. Treatment of type B insulin resistance: a novel approach to reduce insulin receptor autoantibodies. J Clin Endocrinol Metab 2010 Aug;95(8):3641-7.PM:20484479
(299) Palmieri G, Lastoria S, Colao A, Vergara E, Varrella P, Biondi E, et al. Successful treatment of a patient with a thymoma and pure red-cell aplasia with octreotide and prednisone. N Engl J Med 1997;336(4):263-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8995089
(300) Marx A, Wilisch A, Schultz A, Gattenlohner S, Nenninger R, Muller-Hermelink HK. Pathogenesis of myasthenia gravis. Virchows Arch 1997;430:355-64.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9174625
(301) Mygland A, Aarli JA, Matre R, Gilhus NE. Ryanodine receptor antibodies related to severity of thymoma associated myasthenia gravis. J Neurol Neurosurg Psychiatr 1995;57:843-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8021674
(302) Inada K, Okumura M, Shiono H, Inoue M, Kadota Y, Ohta M, et al. Role of positive selection of thymoma-associated T cells in the pathogenesis of myasthenia gravis. J Surg Res 2005 Jun 1;126(1):34-40.http://www.ncbi.nlm.nih.gov/pubmed/15916972?ordinalpos=8&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(303) Kondo K, Monden Y. Thymoma and myasthenia gravis: a clinical study of 1,089 patients from Japan. Ann Thorac Surg 2005 Jan;79(1):219-24.http://www.ncbi.nlm.nih.gov/pubmed/15620947?ordinalpos=12&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(304) Suzuki S, Shimoda M, Kawamura M, Sato H, Nogawa S, Tanaka K, et al. Myasthenia gravis accompanied by alopecia areata: clinical and immunogenetic aspects. Eur J Neurol 2005 Jul;12(7):566-70.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15958099
(305) Geuder KI, Marx A, Witzemann V, Schalke B, Kirchner T, Müller-Hermelink HK. Genomic organization and lack of transcription of the nicotinic acetylcholine receptor subunit genes in myasthenia gravis-associated thymoma. Lab Invest 1992;452:458.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1583885
(306) Offerhaus GJ, Schipper ME, Lazenby AJ, Montgomery E, Morsink FH, Bende RJ, et al. Graft-versus-host-like disease complicating thymoma: lack of AIRE expression as a cause of non-hereditary autoimmunity? Immunol Lett 2007 Nov 30;114(1):31-7.http://www.ncbi.nlm.nih.gov/pubmed/17928069?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(307) Strobel P, Murumagi A, Klein R, Luster M, Lahti M, Krohn K, et al. Deficiency of the autoimmune regulator AIRE in thymomas is insufficient to elicit autoimmune polyendocrinopathy syndrome type 1 (APS-1). J Pathol 2007 Apr;211(5):563-71.http://www.ncbi.nlm.nih.gov/pubmed/17334980?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(308) Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y. Thymoma and autoimmunity. Cell Mol Immunol 2011 May;8(3):199-202.PM:21317916
(309) Amiel LL, Machover D, Droz JP. Dyscrasie plasmocytaire avec arteriopathie, polyneuropathie, syndrome endocrinien. Ann Intern Med 1975;745:749.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1239972
(310) Kim DE, Kim HJ, Kim YA, Lee KW. Kaposi's sarcoma herpesvirus-associated Castleman's disease with POEMS syndrome. Muscle Nerve 2000 Mar;23(3):436-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10679723
(311) Dispenzieri A, Moreno-Aspitia A, Suarez GA, Lacy MQ, Colon-Otero G, Tefferi A, et al. Peripheral blood stem cell transplantation in 16 patients with POEMS syndrome, and a review of the literature. Blood 2004 Nov 15;104(10):3400-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15280195
(312) Watanabe O, Maruyama I, Arimura K, Kitajima I, Arimura H, Hanatani M, et al. Overproduction of vascular endothelial growth factor/vascular permeability factor is causative in Crow-Fukase (POEMS) syndrome. Muscle Nerve 1998 Nov;21(11):1390-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9771661
(313) Soubrier M, Dubost JJ, Serre AF, Ristori JM, Sauvezie B, Cathebras P, et al. Growth factors in POEMS syndrome: evidence for a marked increase in circulating vascular endothelial growth factor. Arthritis Rheum 1997;40:786-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9125266
(314) Dietrich PY, Duchosal MA. Bevacizumab therapy before autologous stem-cell transplantation for POEMS syndrome. Ann Oncol 2008 Mar;19(3):595.http://www.ncbi.nlm.nih.gov/pubmed/18209011?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(315) Kuwabara S, Misawa S, Kanai K, Sawai S, Hattori T, Nishimura M, et al. Thalidomide reduces serum VEGF levels and improves peripheral neuropathy in POEMS syndrome. J Neurol Neurosurg Psychiatry 2008 May 9;.http://www.ncbi.nlm.nih.gov/pubmed/18469028?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(316) Cavaco B, Uchigata Y, Porto T, Amparo-Santos M, Sobrinho L, Leite V. Hypoglycaemia due to insulin autoimmune syndrome: report of two cases with characterisation of HLA alleles and insulin autoantibodies. Eur J Endocrinol 2001 Sep;145(3):311-6.http://www.ncbi.nlm.nih.gov/pubmed/11517012?ordinalpos=13&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(317) Scully RE, Mark EJ, McNeely WF, McNeely BU. Case records of the Massachusetts General Hospital: Case 34-1987. N Engl J Med 1987;317:493-501.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9197219
(318) Rabinowe SL, Rubin IL, George KL, Adri MN, Eisenbarth GS. Trisomy 21 (Down's syndrome): autoimmunity, aging and monoclonal-antibody defined T-cell abnormalities. J Autoimmun 1989;2:25-30.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2526634
(319) Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 1998;20:143-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9771706
(320) Barrett TG, Bundey SE, Macleod AF. Neurodegenration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet 1995;346:1458-63.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7490992
(321) Karasik A, O'Hara C, Srikanta S, Swift M, et al. Genetically programmed selective islet beta-cell loss in diabetic subjects with Wolfram's syndrome. Diab care 1989;12:135-8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2649325
(322) Ou D, Jonsen LA, Metzger DL, Tingle AJ. CD4+ and CD8+ T-cell clones from congenital rubella syndrome patients with IDDM recognize overlapping GAD65 protein epitopes. Implications for HLA class I and II allelic linkage to disease susceptibility. Hum Immunol 1999 Aug;60(8):652-64.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10439311
(323) Rabinowe SL, George KL, Loughlin R, Soeldner JS, Eisenbarth GS. Congenital rubella. Monoclonal antibody-defined T cell abnormalities in young adults. Am J Med 1986;81(5):779-82.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=3490785
(324) Berio A, Piazzi A. A case of Kearns-Sayre syndrome with autoimmune thyroiditis and possible Hashimoto encephalopathy. Panminerva Med 2002 Sep;44(3):265-9.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12094144
(325) Katsanos KH, Elisaf M, Bairaktari E, Tsianos EV. Severe hypomagnesemia and hypoparathyroidism in Kearns-Sayre syndrome. Am J Nephrol 2001 Mar;21(2):150-3.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11359024
(326) Artuch R, Pavia C, Playan A, Vilaseca MA, Colomer J, Valls C, et al. Multiple endocrine involvement in two pediatric patients with Kearns-Sayre syndrome. Horm Res 1998;50(2):99-104.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9701704
(327) Yamashita S, Nishino I, Nonaka I, Goto Y. Genotype and phenotype analyses in 136 patients with single large-scale mitochondrial DNA deletions. J Hum Genet 2008;53(7):598-606.http://www.ncbi.nlm.nih.gov/pubmed/18414780?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(328) Remes AM, Majamaa-Voltti K, Karppa M, Moilanen JS, Uimonen S, Helander H, et al. Prevalence of large-scale mitochondrial DNA deletions in an adult Finnish population. Semin Neurol 2005 Mar 22;64(6):976-81.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15781811
(329) Pennings RJ, Dikkeschei LD, Cremers CW, van den Ouweland JM. [From gene to disease; mutations in the WFS1-gene as the cause of juvenile type I diabetes mellitus with optic atrophy (Wolfram syndrome)]. Ned Tijdschr Geneeskd 2002 May 25;146(21):985-7.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12058630
(330) Yamaguchi S, Ishihara H, Tamura A, Yamada T, Takahashi R, Takei D, et al. Endoplasmic reticulum stress and N-glycosylation modulate expression of WFS1 protein. Biochem Biophys Res Commun 2004 Dec 3;325(1):250-6.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15522226
(331) Viskari H, Paronen J, Keskinen P, Simell S, Zawilinska B, Zgorniak-Nowosielska I, et al. Humoral beta-cell autoimmunity is rare in patients with the congenital rubella syndrome. Clin Exp Immunol 2003 Sep;133(3):378-83.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12930364
(332) Karounos DG, Wolinsky JS, Thomas JW. Monoclonal antibody to rubella virus capsid protein recognizes a b-cell antigen. J Immunol 1993;150:3080-5.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8454875
(333) Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974;2(7892):1279-83.http://www.ncbi.nlm.nih.gov/pubmed/4139522?ordinalpos=45&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(334) Weetman AP. Determinants of autoimmune thyroid disease. Nat Immunol 2001 Sep;2(9):769-70.PM:11526381
(335) Benoist C, Mathis D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol 2001 Sep;2(9):797-801.http://www.ncbi.nlm.nih.gov/pubmed/11526389?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum